
Doctor at the National Health Services (NHS), United Kingdom (UK)
Clostridium genus, a gram-positive, spore-forming bacterium is responsible for causing botulism. BTX was initially discovered by the Belgian scientist Van Ermengem in 1897 and subsequently classified into 7 subtypes (A-G)1. Among these subtypes, botulinum neurotoxin type A (BoNT-A) has become one of the most popular toxin types used in aesthetic medicine due to its stability and strong binding ability1. Furthermore, BoNT-A injections are the most frequently performed aesthetic procedure globally2 since BoNT-A injections are preferred over the surgical procedures due to their affordability and satisfying results3. However, the therapeutic effects of BoNT-A often diminish over time, thereby, repeated injections are often required to maintain the treatment effects4.
Furthermore, repeated injections of BoNT-A may stimulate antibody formation, particularly the neutralising antibodies (NAbs), which counteract the biological activity of BoNT-A. In addition, NAb formation may trigger a partial or a complete nonresponse to further BoNT-A treatment, also known as immunoresistance, thus potentially carries a long-term implication for future therapeutic options4. Notably, most individuals receiving BoNT-A treatments often start at a younger age which may increase their overall lifetime exposure and thus increases their risk of developing immunoresistance to BoNT-A formulations. Therefore, we shall highlight the issues related to immunoresistance and discuss the recommendations made by experts in aesthetic medicine on ways to reduce immunoresistance with BoNT-A treatment.
With the increased usage of BoNT-A, there are growing concerns of secondary nonresponse (SNR), characterised by an initial benefit in the symptoms, followed by a loss of response with subsequent injection at some point of time after BoNT-A treatment4,5. Notably, the BoNT-A SNR may perhaps be related to the neutralising antibody (Nab) formation or other factors (inadequate dosage, incorrect muscle target or improper injection techniques). The tell-tale signs of SNR include dose or interval creeps, where a higher dose of BoNT-A or shorter injection intervals are required to achieve the desired therapeutic effects. However, these signs can be easily overlooked, and may remain under-recognised by the aesthetic practitioners4. So, how common is NAb with BoNT-A use? According to clinical studies, the NAb associated with BoNT-A for therapeutic applications ranges from 0.3% to 27.6%6.
Furthermore, recent studies have reported an increasing incidence of NAbs with certain formulations of BoNT-A, particularly the onabotulinumtoxinA (ONA) at 5.3%4. But the question remains: how many repetitive BoNT injections are required for secondary treatment failure (SFR) and SNR to occur? In some cases, SFR may occur even after one to three injections6.
It is important to note that the introduction of NAbs and antigenicity related to BoNT preparation is dependent on the content of BoNT-A since the different formulation contains different complex proteins. Among the complex proteins, the haemagglutinin-33 (HA-33) is believed to act as an adjuvant and enhances the immune response to BoNT-A injection6.
Additionally, different BoNT-A formulations have different content of albumin and flagellin. Therefore, the percentage of biologically inactive but immunologically active BoNT-A molecule fragments remain different with each formulation. For instance, the incobotulinumtoxin (INCO) formulation contains no biologically inactive fragments, and the total clostridial protein content is reduced from 100 units (U) to 0.44 nanogram (ng). These properties of INCO have been shown to confer to a lower antigenicity compared to other BoNT-A formulations6.
These findings were also substantiated by a cross-sectional clinical study performed by Hefter et al., (2020) in 59 patients with either cervical dystonia, spasticity after stroke (SPAS), severe Meige syndrome and/or oropharyngeal or oromandibular dystonia (ODT) having exclusively treated with INCO (monogroup) and 32 patients with similar clinical conditions treated with other BoNT-A preparation for <9 times, followed by at least 14 sessions of INCO treatment (switch group). Treatment efficacy was assessed using the visual analogue scale (0-100), and the prevalence of NAbs was tested using the mouse hemi-diaphragm assay (MHDA). Astonishingly, none of the patients in the monogroup and only two in the switch group recorded a positive MHDA test.
Moreover, across all indications and patients, mean improvement exceeded 67%6. Most importantly, the improvements reported were not age, gender or change in dose or duration of treatment dependent. The study concluded that INCO treatment conferred a lower antigenicity compared to other BoNT-A formulation. In addition, authors suggested on using the neurotoxin preparation with the lowest amount of neurotoxin (complex) proteins to minimise the risk of developing NAbs against the BoNT-A formulations6.
The immune system activation by BoNT-A is typically controlled by two crucial steps, both of which are necessary to stimulate the classical T-helper cell-dependent antibody production. During the first step, the Toll-like receptors (TLRs) on dendritic cells (DCs) determine if the antigen is potentially dangerous through recognition of characteristic microbial cell surface features (flagellin) and trigger the ¡§danger signals.¡¨ This activates the process of phagocytosis (cellular ingestion and elimination of foreign particles) by the DCs, which then migrate to the lymph nodes to act as antigen presenting cells (APCs)4. The APCs then present the foreign peptide antigen to naïve T-helper cells (nTHC) while providing co-stimulatory signals for the clonal expansion of antigen-specific T-cells. The second step involves antigen-specific T-cells recognising the presented antigen peptide as foreign and trigger clonal expansion. Moreover, they also assist in the activation of antigen-specific B-cells, which then produce antibodies against the original antigen present to the DCs (Figure 1A and Figure 1B)4.
Notably, the BoNT-A complex comprises of a core of 150 kilodalton (kDa) and various neurotoxin-associated proteins (NAPs), as well as non-neurotoxin-associated proteins (non-NAPs). Among the FDA-approved formulations, ONA and abobotulinumtoxinA (ABO) are known to include NAPs and other unnecessary bacterial proteins, whereas INCO contains only the core 150-kDA formulation (Figure 1C)4.
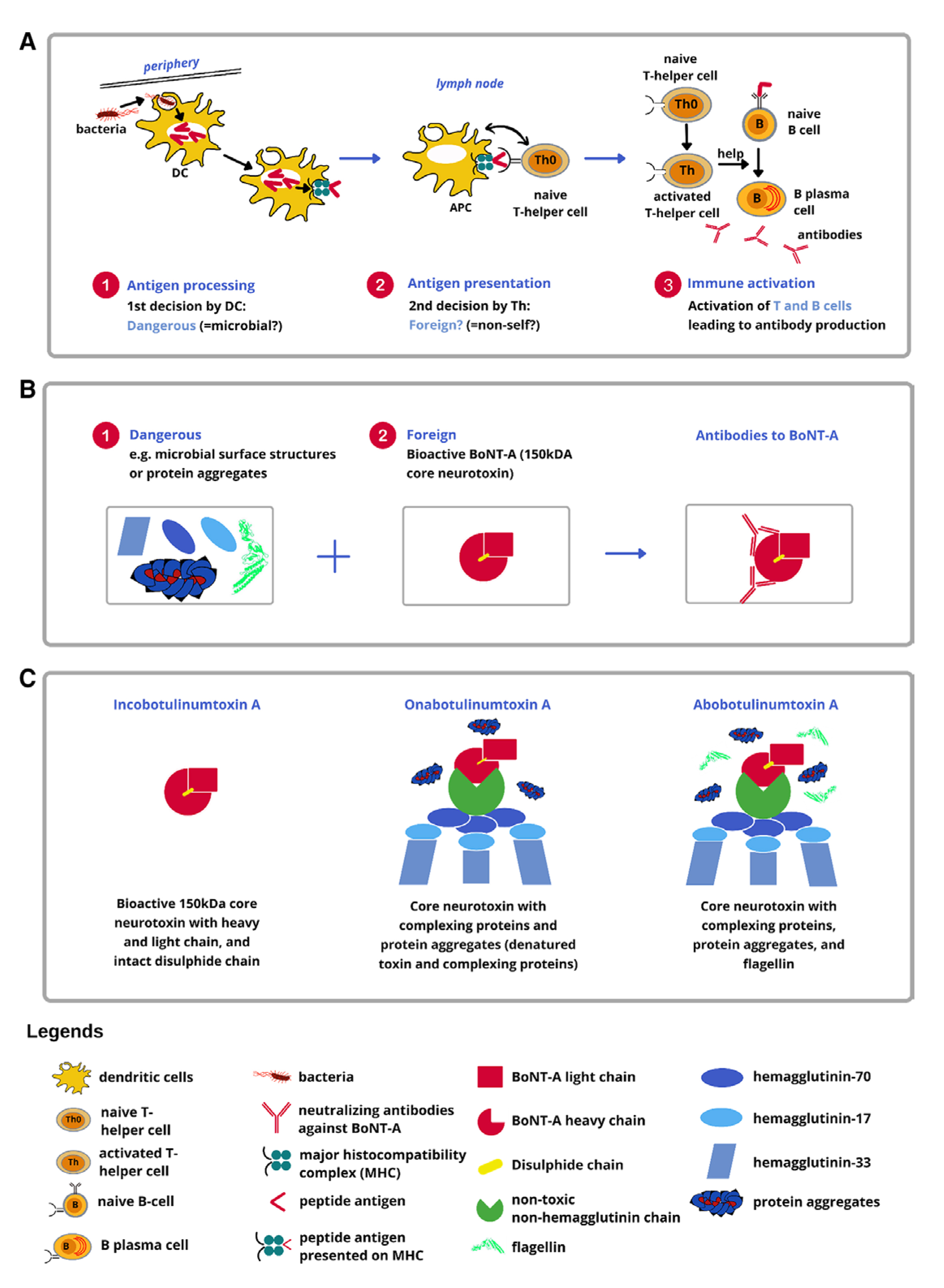 Figure 1. BoNT-A treatment from the immunological perspective. A, Dangerous + foreign? Two key steps controlling the immune response to biologics. The first step involves DCs that determine whether a particle (eg, a microbe) is likely to be ¡§dangerous.¡¨ DCs can recognise microbial surface molecules (eg, flagellin) as ¡§danger signals.¡¨ Upon recognition of microbial danger signals, DCs will be activated and phagocytose the particle bearing the danger signal. Subsequently, these activated DCs migrate to lymph nodes and become professional APCs. The second step involves naive T-helper cells that determine whether a particle is self or foreign. Upon encountering foreign antigen peptides presented by APCs along with co-stimulatory signals, naive T-helper cells become activated and undergo clonal expansion, leading to activation and clonal expansion of antigen-specific B cells. These mature into plasma cells that produce antibodies specific to the antigen that triggered the immune response. B, Development of BoNT-A neutralising antibodies. C, Composition of FDA-approved BoNT-A formulations4.
Figure 1. BoNT-A treatment from the immunological perspective. A, Dangerous + foreign? Two key steps controlling the immune response to biologics. The first step involves DCs that determine whether a particle (eg, a microbe) is likely to be ¡§dangerous.¡¨ DCs can recognise microbial surface molecules (eg, flagellin) as ¡§danger signals.¡¨ Upon recognition of microbial danger signals, DCs will be activated and phagocytose the particle bearing the danger signal. Subsequently, these activated DCs migrate to lymph nodes and become professional APCs. The second step involves naive T-helper cells that determine whether a particle is self or foreign. Upon encountering foreign antigen peptides presented by APCs along with co-stimulatory signals, naive T-helper cells become activated and undergo clonal expansion, leading to activation and clonal expansion of antigen-specific B cells. These mature into plasma cells that produce antibodies specific to the antigen that triggered the immune response. B, Development of BoNT-A neutralising antibodies. C, Composition of FDA-approved BoNT-A formulations4.
In addition, the peptides derived from the BoNT-A core neurotoxin subunit could be identified as foreign by the naïve T-helper cells. However, pure bioactive 150-kDA core neurotoxin often lacks the concomitant danger signals required to activate DCs to become APCs. Without these signals, the first step related to the immune system activation is not registered, allowing the BoNT-A to escape the immune cascade4. In contrast, NAPs such as HA-33 and other bacterial contaminants, particularly flagellin, as well as inactive/denatured toxins and clostridial DNA, may trigger an immune response. In fact, HA-33 has shown to be an immune response stimulator, whereas flagellin and clostridial DNA are adjuvants that readily binds to the TLR-5 and TLR-9 on DCs, respectively, activating the immune cascade. In the context of BoNT-A treatment, NAPs have no therapeutic role and merely enhance the immunogenicity of the injected product. Hence, high doses or repeated injections predispose individuals to ¡§dangerous and foreign¡¨ material¡¨4. Thus, a risk-based approach should be implemented4 when these products are used for aesthetic purposes.
To further understand the implications of immunoresistance to BoNT-A in contemporary local aesthetic practice, a round table discussion involving a panel of nine local aesthetic practitioners and international experts in Hong Kong discussed the implications and impact of immunoresistance to BoNT-A in contemporary aesthetic practice, along with practical strategies for the risk management7. The aim was to evaluate the clinical evidence with real-world data from an Asia-Pacific consumer study on BoNT-A user perception, and experience related to BoNT-A immunoresistance. The panel discussed the analysis from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database on diminishing efficacy of BoNT-A treatment from March 2014 to June 20197. The database identified 23,789 reports of diminishing efficacy, with most reported cases related to ONA (84.6%) and ABO (10.2%) formulations, in contrast to INCO administration (5.3%). In addition, NAb-related SNR was not prevalent in patients who used the INCO formulations compared to other BoNT-A formulations.
The conclusion drawn from these analyses was that there was a higher prevalence of immunoresistance to BoNT-A formulations in real-world settings than in clinical trials7. Additionally, the survey-based studies conducted in the Asia-Pacific region in 2018 (six countries/territories) and in 2021 (eight countries/territories) suggested that most respondents reported being aware of the concept of diminishing treatment efficacy and were able to identify the signs and symptoms associated with diminishing treatment efficacy (Figure 2)7.
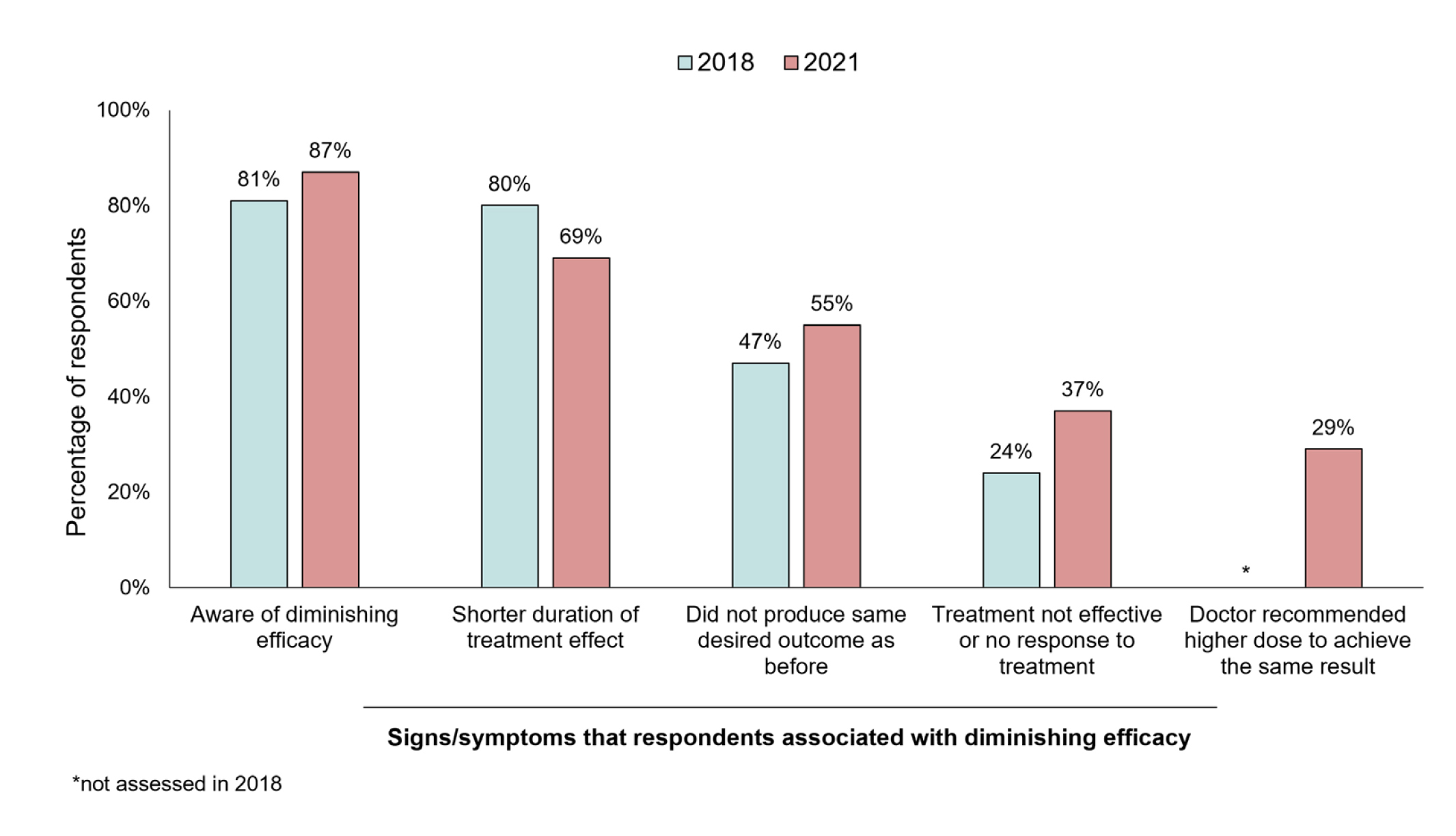
Figure 2. Consumer awareness and experience of diminishing BoNT-A treatment efficacy in 2018 and 2021 Asia-Pacific studies7.
Interestingly, over half of respondents (69% in 2018 and 79% in 2021) reported they had experienced diminishing efficacy7. The results also illustrated the disappointment expressed by the respondents secondary to the diminishing efficacy of BoNT-A, with more than one-third participants felt anxious, sad, or frustrated. However, the emotional impact of BoNT-A immunoresistance did not deter them from continuing the treatment. Of note, a much lower proportion of respondents (60% in the 2021 study and 36% in the 2018 study) were aware that the presence of impurities such as complex proteins may increase their risk of developing NAb.
In light of these findings, the panel advocated that healthcare professionals (HCPs) should communicate with their patients regarding the cause and consequences of toxin resistance in ways that is easier for their patients to understand7. Furthermore, the panel also recommended that the HCPs should consider investigating the cause of diminished efficacy in each patient before recommending treatment changes7. Not surprisingly, similar trends were also reported locally, with only 55% of patients acknowledging to understand the key driving factors related to the development of NAbs against BoNT-A formulations.
Thus, the panel concluded that the HCPs should recognise the immunogenicity as a potential complication related to BoNT-A treatment and should remain vigilant regarding this phenomenon (Figure 3)7. They also encouraged the HCPs to spend more time with their patients during the consultation to establish the treatment histories, in addition to educating their patients on the adverse effects of the treatment as well as to consider using BoNT-A formulation with the lowest immunogenicity from the beginning. These may then help to minimise the risk of immunoresistance7. In conclusion, immunoresistance remains a clinical challenge in aesthetic community and thereby, requires serious attention in order to minimise the patient risk and improve the treatment outcomes.
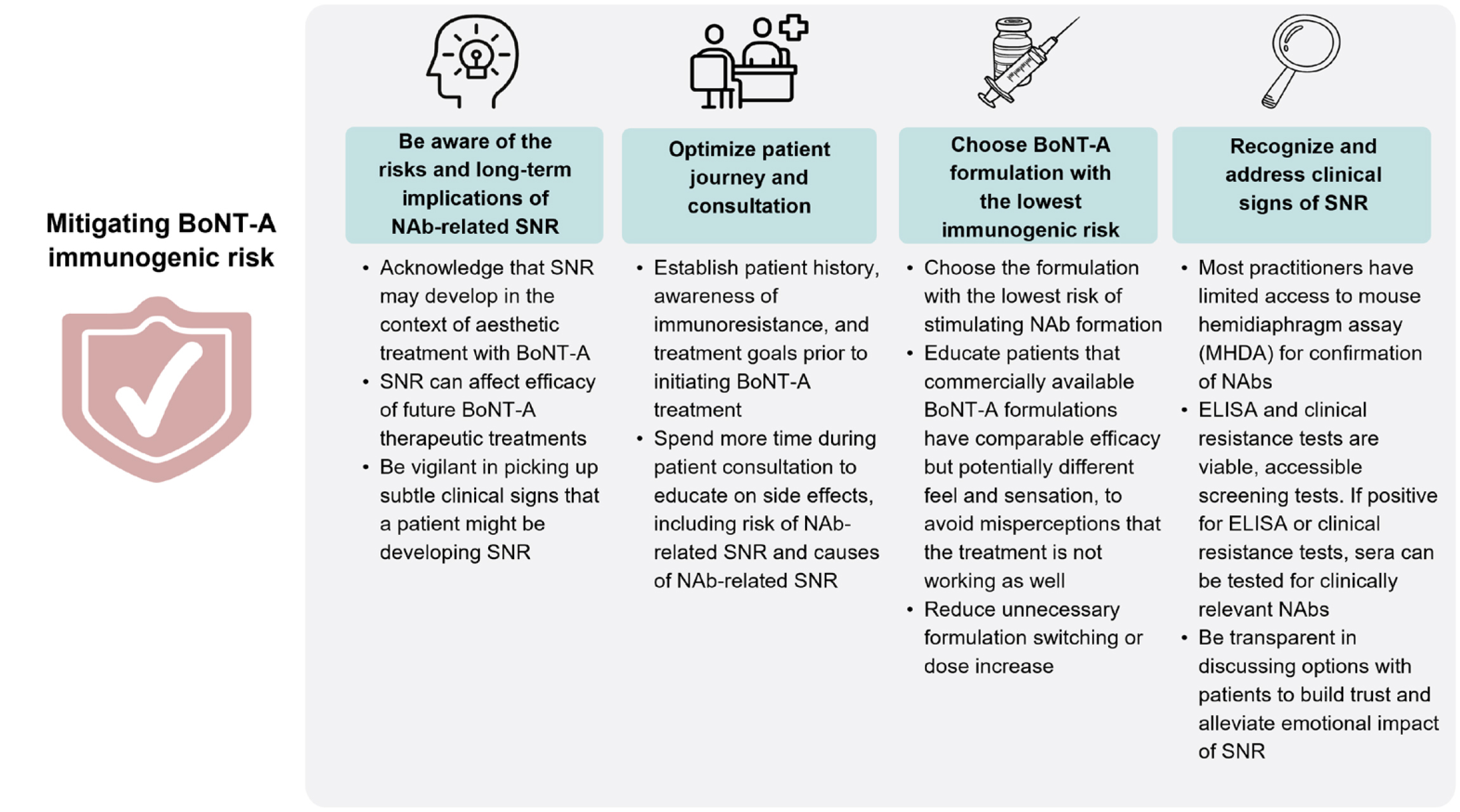 Figure 3. Preferred strategies to mitigate immunogenic risk with BoNT-A treatment7. BoNT-A= botulinum neurotoxin A; Nab= neutralising antibody; SNR= secondary non-response.
Figure 3. Preferred strategies to mitigate immunogenic risk with BoNT-A treatment7. BoNT-A= botulinum neurotoxin A; Nab= neutralising antibody; SNR= secondary non-response.
References
1. Li TT, et al. Medicine (Baltimore) 2023; 102(30): e34461. 2. Ho WWS, et al. Toxins 2023; 15(7): 456. 3. Zargaran D, et al. Aesthet Surg J 2022; 42(5): Np327-np36. 4. Ho WWS, et al. Plast Reconstr Surg Glob Open 2022; 10(6): e4407. 5. Bellows S, et al. Toxins (Basel) 2019; 11(9). 6. Hefter H, et al. J Neurol 2020; 267(5): 1340-7. 7. Ho WWS, et al. Toxins (Basel) 2023; 15(7).





