

Specialist in Endocrinology, Diabetes and Metabolism
The COVID-19 pandemic has led to substantial morbidity and mortality globally. Patients with diabetes are highly susceptible to adverse outcomes and complications of COVID-19 infection, whereas COVID-19 infection disrupts glucose regulation, rendering glycemic control difficult1. Given the COVID-19 pandemic has now persisted for over 2 years, there is growing evidence suggesting that people infected with COVID-19 could experience a wide range of post-acute sequelae, referred to as “long COVID”2. While complications of diabetes have been widely reported during the acute phase of COVID-19, less is known about the risk and burden of diabetes and related outcomes in the post-acute phase of COVID-19. To uncover the vicious cycle between diabetes and COVID-19, as well as the impacts of its sequelae, Dr. Wong Cheuk Lik, Specialist in Endocrinology, Diabetes and Metabolism, shared his insight on the pathophysiology of diabetes complicated with COVID-19 infection and opinions on related clinical issues.
When Diabetes Meets COVID-19
Former epidemiological studies have identified diabetes as one of the major risk factors for severe complications and mortality in people infected with COVID-193. The potential pathogenetic mechanisms underlying poor clinical outcomes in patients with diabetes due to COVID-19 infection include immune dysfunction, exacerbation of pre-existing low-grade inflammation, insulin resistance, hyperglycemia and dysregulated renin-angiotensin-aldosterone system (RAAS). Defective immune functions, including impaired natural killer (NK) cell function and adaptive cellular immunity, reduced chemotaxis and phagocytosis in polymorphs, insufficient type 1 interferon (IFN) response and complement defects predispose subjects with diabetes to severe infection4. On the other hand, diabetes is characterised by a chronic low-grade pro-inflammatory state contributed by T-helper 1 (Th1) and T-helper 2 (Th2) imbalance. This pro-inflammatory state can be markedly accentuated by overactivated T cells and macrophages during COVID-19 infection, and the resultant over-production of cytokines may trigger cytokine storm, leading to vascular endothelial dysfunction, diffuse alveolar damage, acute lung injury, multiple systemic coagulation and multi-organ failure. Over-production of cytokines, such as interleukin (IL)-1, IL-6 and tumor necrosis factor-alpha (TNF-), also induces a state of insulin resistance and potentially causes hyperglycemia, both of which contribute to endothelial damage and perpetuate the pro-inflammatory and pro-coagulant cycle5,6. In addition, hyperglycemia enhances SARS-CoV-2 replication in monocytes and augments monocyte pro-inflammatory cytokine response. Taken the effect of sustained viral proliferation via glycolysis together, hyperglycemia has been well recognized as an important risk factor for severe complications7,8.
In addition, Dr. Wong highlighted that the entry of SARS-CoV-2 virus may be facilitated by an increase of angiotensin-converting enzyme 2 (ACE2) receptor expression and furin in diabetes and state of hyperinsulinemia, which is further enhanced by the surface glycoprotein dipeptidyl peptidase-4 (DPP4). ACE2, a homologue of angiotensin-converting enzyme (ACE), normally hydrolyses angiotensin-II (Ang II) to angiotensin-(1–7), which counteracts the negative effects of the RAAS and exerts anti-inflammatory effects. SARS-CoV-2 infection downregulates ACE2 expression on cells, thereby disrupting the physiological balance between ACE/ACE2 and Ang-II/angiotensin-(1–7) and subsequently leading to severe inflammatory response, cardiovascular (CV) events, increased thromboembolic risk, disseminated intravascular coagulation (DIC) and severe organ injury9–11. Last but not least, diabetes is often associated with a wide range of comorbidities such as obesity, pre-existing CV disease, chronic kidney disease, hypertension and heart failure, which are also risk factors for severe COVID-19 infection12. According to the CORONADO study, microvascular and macrovascular complications of diabetes mellitus were significantly associated with increased risk of mortality in patients with COVID-1913. A summary of the potential pathogenic mechanisms of severe COVID-19 infection in patients with diabetes is illustrated in Figure 1.
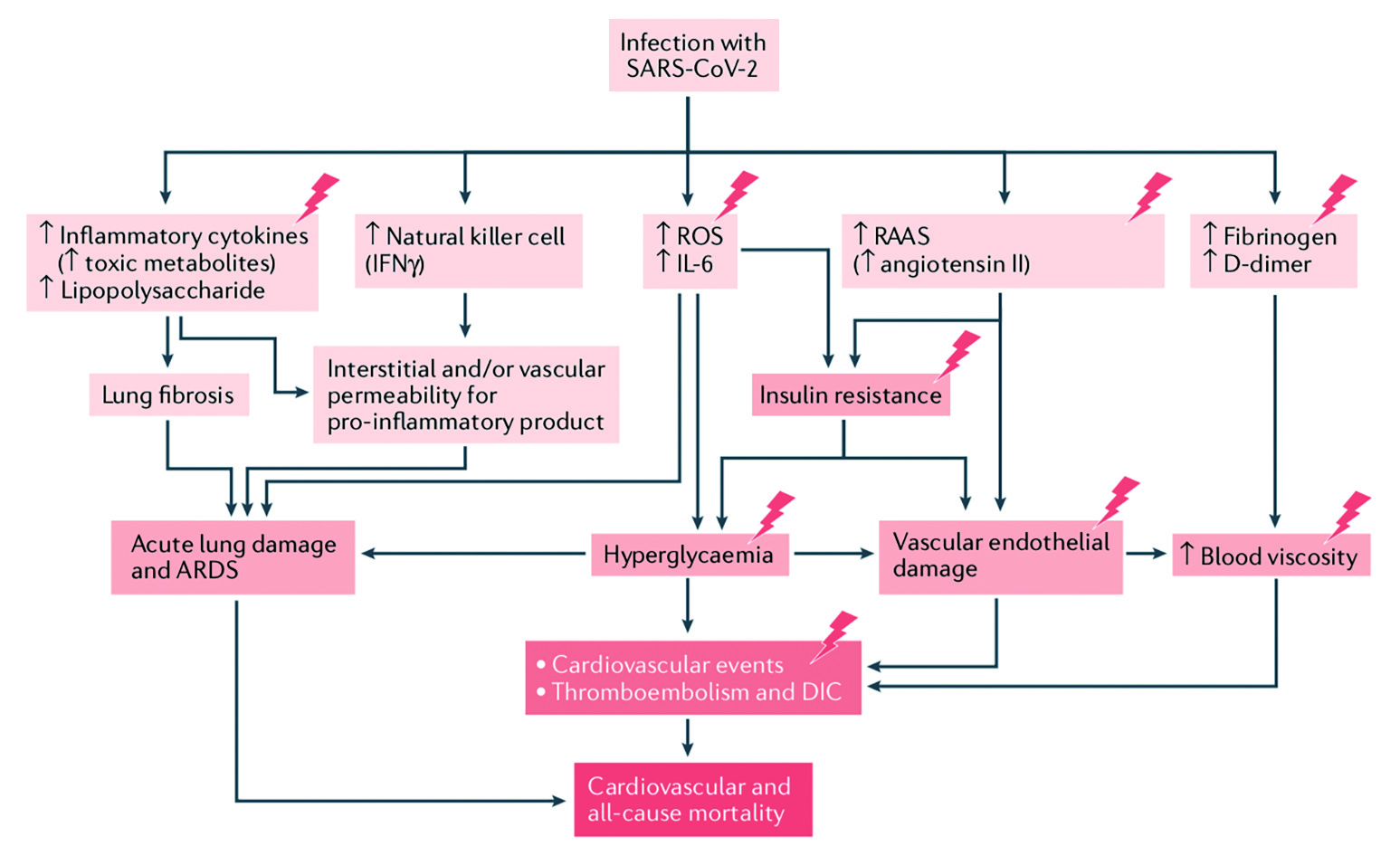
Figure 1. Potential pathogenic mechanisms in patients with diabetes and COVID-19, lightning bolts indicate mechanisms that are accentuated in patients with diabetes, ARDS: acute respiratory distress syndrome (adapted from Lim et al)14
A Closer Look at the Subtypes of Diabetes
In evaluating the impact of COVID-19 specifically in type 1 diabetes (T1D) and type 2 diabetes (T2D), Dr. Wong noted that an increased risk of severe COVID-19 was reported in both types of diabetes as compared to non-diabetic individuals, with probably higher risk for adverse COVID-19-related outcomes in T1D. A prospective cohort study in US involving 6,138, 40, and 273 patients without diabetes, with type 1 and type 2 diabetes showed that people with type 1 diabetes had adjusted odds ratios of 3.90 (95% CI: 1.75–8.69) for hospitalization and 3.35 (95% CI: 1.53–7.33) for greater illness severity, which was similar to risk in type 2 diabetes15. In contrast, a population-based study from the United Kingdom (UK) demonstrated that out of 23,804 COVID-19-related in-hospital deaths, unadjusted 72-day mortality rates per 100,000 people were 27 for individuals without diabetes, 138 for T1D and 260 for T2D. Interestingly, after adjustment for age, sex, socioeconomic deprivation, ethnicity and geographical region, the odd ratio (OR) for COVID-19-related mortality was even higher in patients with T1D compared to patients with T2D (3.51 vs 2.03)16. The impact of obesity also differed in patients with T1D and T2D with elevated BMI in T1DM associated with additional risk of mortality as compared to T2D17.
On the other hand, while pregnant women with COVID-19 and gestational diabetes (GDM) are 3.3 times more likely to be admitted to the intensive care unit (ICU) than women without pre-existing diabetes/GDM18, GDM as a whole does not seem to increase the risk of severe COVID-19 outcomes. However, Dr. Wong pointed out that increased risk of adverse maternal and neonatal outcomes (a composite of admission to ICU, viral pneumonia, oxygen supplementation or maternal mortality in mothers, and stillbirth, neonatal death or transfer to neonatal ICU in neonates) has been observed in a subset of pregnant women with GDM who were also overweight or obese pre-conceptually, and who required insulin treatment. Furthermore, adverse maternal outcomes were more common in women when COVID-19 was diagnosed with or shortly after the diagnosis of GDM than those with COVID-19 diagnosis before GDM diagnosis18. Nevertheless, pregnant women with COVID-19 infection are already a vulnerable group of patients at increased risk of severe clinical course and adverse pregnancy outcomes, especially if they are unvaccinated19. If hospitalisation for COVID-19 is indicated for a pregnant patient, close monitoring and care should be provided in a facility that can conduct maternal and fetal monitoring, when appropriate.
The Vicious Cycle
As discussed, pre-existing diabetes is a risk factor for poor outcomes and mortality upon COVID-19 infection, whereas published data have suggested that the infection can lead to a deterioration in glycemic control. Apart from the pathogenic links leading to hyperglycaemia mentioned above, Dr. Wong added that the use of systemic glucocorticoids in managing COVID-19 infection might impair glycemic control as well. Glucocorticoids are widely used as potent anti-inflammatory and immunosuppressive agents to treat a wide range of diseases, and the RECOVERY trial showed that dexamethasone at a dose of 6mg daily up to 10 days lowered 28-day mortality among patients hospitalised with COVID-19 who were receiving either invasive mechanical ventilation or oxygen therapy at randomisation20. However, this medication has been reported to induce insulin resistance, and can provoke severely uncontrolled hyperglycemia in patients with known diabetes, or trigger new-onset hyperglycemia in patients without a history of diabetes mellitus, a condition referred to as glucocorticoid-induced diabetes mellitus (GIDM)21.
Insulin deficiency has also been proposed to contribute to new-onset diabetes associated with COVID-19 infection. “SARS-CoV-2 virus may damage pancreatic beta-cell directly causing defect in insulin secretion,” Dr. Wong highlighted. Steenblock et al (2021) showed the presence of SARS-CoV-2 in pancreatic samples from COVID-19 patients which strongly hints to the ability of SARS-CoV-2 to invade the pancreas22. Additionally, as ACE2 plays a crucial role in glucose homoeostasis and insulin secretion by regulating beta cell physiology and is expressed in pancreatic beta-cells, the attachment of SARS-CoV-2 to ACE2 receptors in beta cells could cause acute impairment in insulin secretion. Finally, glucotoxicity as a result of hyperglycemia would further aggravate beta-cell secretory function and worsen insulin deficiency.
In reality, observational studies have demonstrated an increased risk of newly diagnosed diabetes after COVID-19. A recent retrospective cohort analysis involving 35,865 individuals with documented COVID-19 in Germany indicated an increased incidence of T2D amongst individuals with COVID-19 compared with those with acute upper respiratory tract infections (15.8 vs 12.3 per 1000 person-years, Figure 2), with an estimated incidence rate ratios for type 2 diabetes of 1.2823. Moreover, Montefusco et al (2021) reported that glycaemic abnormalities can be detected for at least 2 months in patients who recovered from COVID-1924. Yet, Dr. Wong noted that the impact of COVID-19 on glycaemic control in longer term is still uncertain and is an area of ongoing research.
Managing Diabetes Complicated with COVID-19 Dr. Wong emphasised that COVID-19 patients with diabetes had poor prognosis, and the presence of other comorbidities inflates the risk. Specifically, a study in China showed that in patients with diabetes hospitalised for COVID-19, the presence of two or more of the following abnormalities, including lymphopenia (lymphocyte count ≤0.45×109/L), lactate dehydrogenase >600U/L, high-sensitivity C-reactive protein (hsCRP) >90mg/L, and interleukin (IL)-10 >10U/mL portend high mortality25. Other biomarkers such as D-dimer and NT-pro-BNP, as well as clinical risk scores, have been proposed to predict severity of COVID-1926–28. “For diabetic patients with COVID-19, more attention should be paid to the dynamic monitoring of clinical parameters, inflammatory and thrombotic markers, and the control of hyperglycaemia,” he advised. Of importance, he reminded frontline healthcare providers to closely monitor glycaemia in patients with COVID-19 and optimise glycaemic control as needed. In addition, patients with diabetes are at increased risk of thromboembolic events, and pharmacological thromboprophylaxis (usually in the form of low-molecular weight heparin [LMWH]) should be considered in hospitalised patients with at high thromboembolic risk according to local guidelines, although it has to be acknowledged that the clinical efficacy, the dose and the timing of anticoagulation remain highly controversial given the heterogeneity of the currently available clinical evidence29–31.
As compared to the management of non-diabetic subjects with COVID-19, Dr. Wong reminded that glucose-lowering drugs commonly used to treat diabetes might require adjustment in infected patients with diabetes. While anti-diabetic drugs can usually be continued in mild COVID-19 infection, interruption of some of these agents may be needed to prevent adverse effects in (potentially) severely ill patients. “For instance, metformin may cause lactic acidosis in patients with or at risk of severe sepsis, acute kidney injury, hypoxia, acute cardiac or hepatic failure. Initiation or maintenance of glucagon-like peptide 1 receptor agonists (GLP-1RA), despite their theoretical anti-inflammatory and anti-oxidant properties, is not recommended owing to the risk of gastrointestinal side effects such as nausea and vomiting and resultant risk of aspiration, and they will take time to become effective due to slow up-titration. On the other hand, sodium-glucose cotransporter 2 inhibitors (SGLT2i) might cause ketoacidosis, especially in critically ill patients, and their glucose-lowering effects are limited substantially in the face of a reduced estimated glomerular filtration rate. Similarly, sulphonylurea (SU) and thiazolidinedione (TZD) are associated with risk of hypoglycemia and heart failure respectively, especially in the presence of cardiac and kidney injury14,32,” he said.
In line with Drucker’s recommendation, insulin is the preferred treatment of choice for in-patient hyperglycaemia, especially in critically ill patients33. Indeed, optimal glucose control using insulin infusion has been shown to significantly reduce IL-6 and D-dimer levels and improved disease severity in patients with COVID-19 with or without diabetes mellitus34. Alternatively, DPP4 inhibitors may be used as an adjunct to insulin in selected patients with type 2 diabetes who have milder degrees of hyperglycaemia14,35.
Dr. Wong further addressed that patients with diabetes often have concurrent medical conditions, such as hypertension, hyperlipidemia and ischemic heart disease, requiring multiple medications for optimal control. Potential drug-drug interactions (DDIs) thus have to be taken into account when prescribing treatment for patients with diabetes and COVID-19. One example is the DDI between protease inhibitors and statin, which may necessitate the interruption of the latter during the course of anti-viral treatment. Protease inhibitors have also been reported to increase risk of hyperglycaemia and patients with diabetes and/or hyperglycaemia being treated with these agents should be advised on closer monitoring of blood glucose. The impact of other COVID-19 treatments on metabolism has been extensively reviewed elsewhere and is beyond the scope of this article14.
In considering other major difficulty in managing diabetic patients with COVID-19, Dr. Wong claimed that guidance on restarting patients on optimal anti-diabetic therapy after recovering from COVID-19 is currently lacking. Nevertheless, the immediate post-COVID19 period may provide an opportunity to refine treatment and to counter clinical inertia that predates the pandemic. Furthermore, he emphasised that the optimisation applies not only to glycaemic control, but also other risk factors and co-existing conditions such as hyperlipidemia, kidney disease, heart failure and atherosclerotic cardiovascular diseases.
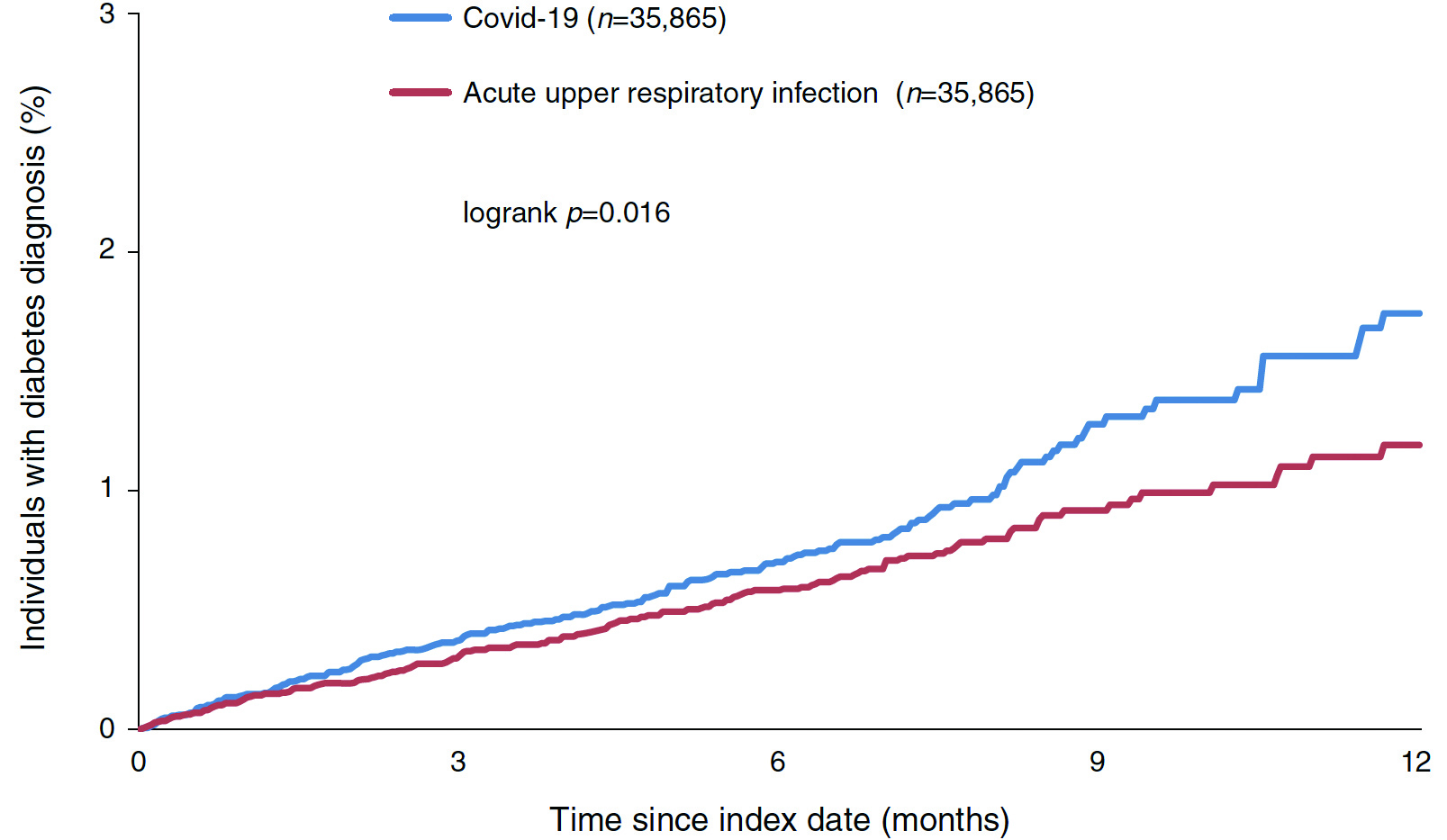
Figure 2. Kaplan-VMeier curves for newly diagnosed T2D in matched individuals with COVID-19 and acute upper respiratory tract infections23
The Sequelae of COVID-19
Patients may suffer from debilitating symptoms developed during or after COVID-19 infection. These symptoms, not explained by an alternative diagnosis, continue for more than 12 weeks after the infection are known as Post COVID-19 Syndrome (PCS), or more commonly long COVID2. Dr. Wong outlined that long COVID-19 Syndrome is an umbrella term comprising both post-acute COVID-19 and PCS, depending on the duration after the acute onset of symptoms36.
Carfi et al (2020) reported that 32% COVID-19 survivors had 1 or 2 COVID-19–related symptoms and 55% had three or more, whereas fatigue (53.1%), dyspnea (43.4%), joint pain (27.3%) and chest pain (21.7%) were the most commonly reported symptoms37. On the other hand, a meta-analysis by Lopez-Leon et al (2021) investigating the long-term effects of COVID-19 revealed that, the five most common symptoms were fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), and dyspnea (24%)38.
Established literature suggested that risk factors for developing long COVID include severe disease during acute COVID-19, pre-existing pulmonary disease, higher body mass index, older age, presence or persistence of symptoms (such as dyspnea, fatigue/muscular weakness, and anxiety or mood disorders), and female sex39.
The Burdens of New-onset Diabetes in Long COVID
It has been reported that people who survived the first 30 days of SARS-CoV-2 infection exhibited an increased risk (hazard ratio [HR]: 1.40) and excess burden of incident diabetes at 12 months, compared with contemporary controls40. Notably, Barrett et al (2022) reported that persons aged <18 years with COVID-19 were more likely to receive a new diabetes diagnosis >30 days after infection than were those without COVID-19 and those with pre-pandemic acute respiratory infections. Additionally, non–SARS-CoV-2 respiratory infection was not associated with an increased risk for diabetes41. However, whether new-onset diabetes will remain permanent is currently not known, as the long-term follow-up of these patients is limited. Nevertheless, it has been reported than about two-thirds of patients diagnosed to have hyperglycemia during admission had recovered euglycaemia42. An international registry has been established to further investigate the complex relationship between new-onset diabetes and COVID-1943.
“The mechanisms behind new-onset diabetes following COVID-19 infection are likely multifaceted. Insulin resistance and beta-cell damage are the two crucial mechanisms that have been proposed by several studies.” Dr. Wong commented. He added that diabetes in the post-acute COVID-19 setting may be the consequences of direct viral injury, immunological and inflammatory damage to pancreatic beta-cells, as well as iatrogenic complications. Nonetheless, while the possible mechanisms explaining the occurrence of new-onset diabetes with SARS-CoV-2 infection during the acute phase have been extensively discussed, it is yet to be determined whether these mechanisms persist in the post-acute phase for the development of new-onset diabetes in long COVID.
Screening for New-onset Diabetes - To Be or Not to Be?
Recent literature suggested that patients with COVID-19 admitted to the ICU and those aged <70 years are at increased risk of new-onset diabetes post-COVID-19 and, thus, may be screened for new-onset diabetes at 3 months after discharge with a fasting plasma glucose, a 2-hour plasma glucose during oral glucose tolerance test or glycated hemoglobin (HbA1C) as per the American Diabetes Association (ADA) Standards of Medical Care in Diabetes. Moreover, patients with COVID-19 with documented in-hospital hyperglycaemia, including steroid-induced hyperglycaemia, but normoglycaemia and off all anti-diabetic drugs at the time of discharge should be re-evaluated at 3 months after discharge. Notably, it is proposed that the dose and number of anti-hyperglycaemic medications should be adjusted as per the glycaemic profile44. This highlights the importance of monitoring glycaemic control and blood glucose profile beyond the acute phase of COVID-19 infection.
Besides, Nalbandian et al (2021) advocated that serologic testing for T1D-associated autoantibodies and post-prandial C-peptide measurements should be obtained at follow-up in patients with newly diagnosed diabetes mellitus in the absence of traditional risk factors for T2D39, although it has to be appreciated that solid evidence linking COVID-19 infection and T1D is currently lacking Alternatively, cases of new-onset T1D have been reported after mRNA-based COVID-19 vaccination in susceptible subjects45.
Prevention is the Best Medicine
In summary, Dr. Wong expressed that, in managing diabetic patients infected with COVID-19, “prevention is always better than treatment”. Optimising diabetic control is crucial as hyperglycaemia and pre-infection HbA1c have been well demonstrated to increase risk of COVID-19 related mortality8. Frontline clinicians should also counsel patients with diabetes of the signs and symptoms of glycaemic deterioration in case they may contract COVID-19. Close monitoring of glucose is needed once a patient is infected, as well as post COVID-19, considering that adjustment of anti-diabetic drugs may be needed in some patients. Last but not least, Dr. Wong stressed that up-to-date COVID-19 vaccination and boosters are of great importance in reducing the risk of severe COVID-19 infection and are strongly recommended in patients with diabetes at all ages. Maintaining good personal hygiene, mask wearing and performing diagnostic testing rapidly if suspected of being infected remain valid at the time of ongoing pandemic.
References
1. Feldman et al. Diabetes 2020; 69: 2549-V65. 2. Raveendran et al. Diabetes Metab Syndr 2021; 15. DOI:10.1016/J.DSX.2021.102235. 3. Smati et al. Curr Diab Rep 2022; 22: 53-V63. 4. Erener. Mol Metab 2020; 39. DOI:10.1016/J.MOLMET.2020.101044. 5. Ragab et al. Front Immunol 2020; 11: 1446. 6. Šestan et al. Immunity 2018; 49: 164-177.e6. 7. Drucker. Cell Metab 2021; 33: 479-V98.
8. Prattichizzo et al. Diabetes Metab Res Rev 2022; 38. DOI:10.1002/DMRR.3476. 9. Shekhar et al. Horm Metab Res 2020; 52: 471. 10. Ni et al. Crit Care 2020; 24: 1-V10. 11. Viswanathan et al. World J Diabetes. 2021 Mar 15; 12(3): 215-V237. 12. Li et al. PLoS One 2021; 16. DOI:10.1371/JOURNAL.PONE.0250602. 13. Cariou et al. Diabetologia 2020; 63: 1500. 14. Lim et al. Nat Rev Endocrinol 2020 171 2020; 17: 11-V30. 15. Gregory et al. Diabetes Care 2021; 44: 526-V32. 16. Barron et al. Lancet Diabetes Endocrinol 2020; 8: 813-V22. 17. Landstra et al. Front Endocrinol (Lausanne) 2021; 12: 599. 18. Kleinwechter et al. Am J Obstet Gynecol 2022; DOI:10.1016/J.AJOG.2022.05.027. 19. Stock et al. Nat Med 2022 283 2022; 28: 504-V12. 20. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021; 384: 693-V704. 21. Suh et al. Endocrinol Metab 2017; 32: 180. 22. Steenblock et al. Nat Commun 2021; 12: 15. 23. Rathmann et al. Diabetologia 2022; 65: 949-V54. 24. Montefusco et al. Nat Metab 2021; 3: 774-V85. 25. Hui et al. PLoS One 2020; 15. DOI:10.1371/JOURNAL.PONE.0243602. 26. Gao et al. Respir Res 2020; 21: 1-V7. 27. Rostami et al. Expert Rev Hematol 2020; 13: 1265-V75. 28. Galloway et al. J Infect 2020; 81: 282. 29. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med 2021; 385: 790-V802. 30. Bradbury et al. Lancet 2022; 399: 5-V7. 31. Flumignan et al. Cochrane database Syst Rev 2020; 10. DOI:10.1002/14651858.CD013739. 32. Kazakou et al. Front Endocrinol (Lausanne) 2022; 13. DOI:10.3389/FENDO.2022.780663. 33. Drucker. Endocr Rev 2020; 41: 457-V69. 34. Sardu et al. Diabetes Care 2020; 43: 1408-V15. 35. Korytkowski et al. J Clin Endocrinol Metab 2022; DOI:10.1210/CLINEM/DGAC278. 36. CDC.Long COVID or Post-COVID Conditions. 2022. 37. Carfì et al. JAMA 2020; 324: 603. 38. Lopez-Leon et al. Sci Rep 2021; 11. DOI:10.1038/S41598-021-95565-8. 39. Nalbandian et al. Nat Med 2021; 27: 601. 40. Narayan et al. Lancet Diabetes Endocrinol 2022; 10: 298-V9. 41. Barrett et al. MMWR Morb Mortal Wkly Rep 2022; 71: 59-V65. 42. Clarke et al. Endocrinology 2022; 163. DOI:10.1210/ENDOCR/BQAB203. 43. Rubino et al. N Engl J Med 2020; 383: 789-V90. 44. Pal et al. Endocr Pract 2022; 28: 425-V32. 45. Sasaki et al. J Diabetes Investig 2022; 13: 1105-V8.





