
Exploring Chemotherapy-associated
Cognitive Impairment in Colorectal Cancer
Chemotherapy, which is commonplace in treating a plethora of cancers, has seen increasing concerns over its possible role in inducing cognitive impairment1. In breast cancer, for instance, more than 50% of patients have raised cognitive complaints after receiving chemotherapy2. Though studies in this area could be dated back to the 1980s, most of which were conducted primarily in breast cancer, and those targeting colorectal cancer were relatively limited1. It is thus worthwhile to explore the potential association between chemotherapy and cognitive impairment in colorectal cancer patients and what the existing studies could entail in daily clinical practice.
Chemotherapy in Colorectal Cancer: The Issue of Toxicity
Despite having been outnumbered by lung cancer recently according to the 2019 Hong Kong Cancer Registry data, colorectal cancer remains one of the most common cancers in Hong Kong3,4. Whereas surgery serves as the mainstay curative treatment for primary colorectal cancer, chemotherapies, such as oxaliplatin and irinotecan, would be required to resect the more aggressive metastatic disease5. The current first-line treatment regimens for the metastatic disease include the FOLFOX or the FOLFIRI protocol, both of which could confer a comparable median overall survival benefit of approximately 20 months5,6. However, these cytotoxic agents are often accompanied with undesirable side effects, where neutropenia and diarrhea are some of the commonly experienced conditions5.
Controversy Remains Owing to Inconsistent Study Findings
Yet, despite these observed patterns underlying the potential contributive role of chemotherapy towards cognitive impairment, contradictory clinical study results exist as to whether these cytotoxic agents will indeed pose harm to the cognitive functions. A prospective interview study conducted by Shaffer VA et al. that aimed to explore whether chemotherapy would accelerate cognitive decline in breast (n=141) and colorectal cancer patients (n=224), whose mean age at diagnosis was 75.5, revealed that, following a median follow-up of 3.1 years after diagnosis, there were no differences in the rates of cognitive decline before and after diagnosis in adjusted models (p=0.86). Likewise, no difference was noted in the rate of cognitive decline post-diagnosis between patients who received chemotherapy and those who did not (p=0.84), rendering no evident association between chemotherapy and cognitive decline in the selected elderly population10.
On the other hand, a meta-analysis of 11 prospective studies, published recently in 2021, unveiled that older patients were more susceptible to chemotherapy-related cognitive impairment (CRCI) than their younger counterparts (β=‒0.016, p<0.001), although the association between chemotherapy and cognitive impairment remained unclear in overall colorectal cancer patients (standardized mean differences=0.003; 95% CI: -0.080 to 0.086)7. Albeit negligible, chemotherapy still correlates positively towards cognitive deterioration, while age, in particular, serves as a critical moderator of CRCI7.
The Consequences of Cognitive Impairment in Colorectal Cancer Patients
The inconsistency in findings could somehow be addressed by the heterogeneity in the recruited sample populations as in different studies, where studies enrolling older subjects would generally be in favor of the said association owing to the subjects’ supposedly reduced baseline cognitive reserves7. Regardless, clinicians should always remain vigilant of this possible correlation whenever chemotherapeutic agents with neurotoxic potential are prescribed, since cognitive impairments, once unfortunately developed, could leave impactful and profound consequences to the sufferers as well as their care-takers, thus jeopardizing their quality of life8. For instance, the inability to stay attentive could adversely affect school, job, and social performances, thereby impeding personal achievements and advancements in these areas8. Not being able to multi-task could also make raising children and maintaining personal relationships uneasy, which would in turn trouble the lives of the dependents and the care-takers8.
Predicting Cognitive Impairment Risk
To avoid having 'chemo-brain’ developed unnoticed in patients, clinicians can recognise some of the risk factors contributing to this condition. Assuringly, Zhou SP et al. have lately published a study indicating that a prediction model can now be developed to help estimate the risk of CRCI in colorectal cancer patients post-chemotherapy11. In the study, 386 colorectal cancer patients who had received chemotherapy were recruited and regression analysis was performed. Three prediction models, namely logistic regression, random forest, and support vector machine models, were developed, which were subsequently assessed by calibration and receiver operating characteristic curves and C-indexes, coupled with a decision curve analysis (DCA) to determine their clinical utility. It was discovered that the logistic regression model conferred the strongest predictive power (AUC=0.799) when 14 different predictive factors, such as age, body-mass-index (BMI), diabetes, and exercise, were included. A visual nomogram prediction model (Figure 1) constructed from the logistic model reflected good predictive ability and strong clinical utility, based on the C-index (0.826) and calibration curve and the DCA curves respectively11.
Clinically, this novel model can serve as a promising screening tool in identifying patients at risk of CRCI and helping to individualize treatments that can best secure their quality of life.
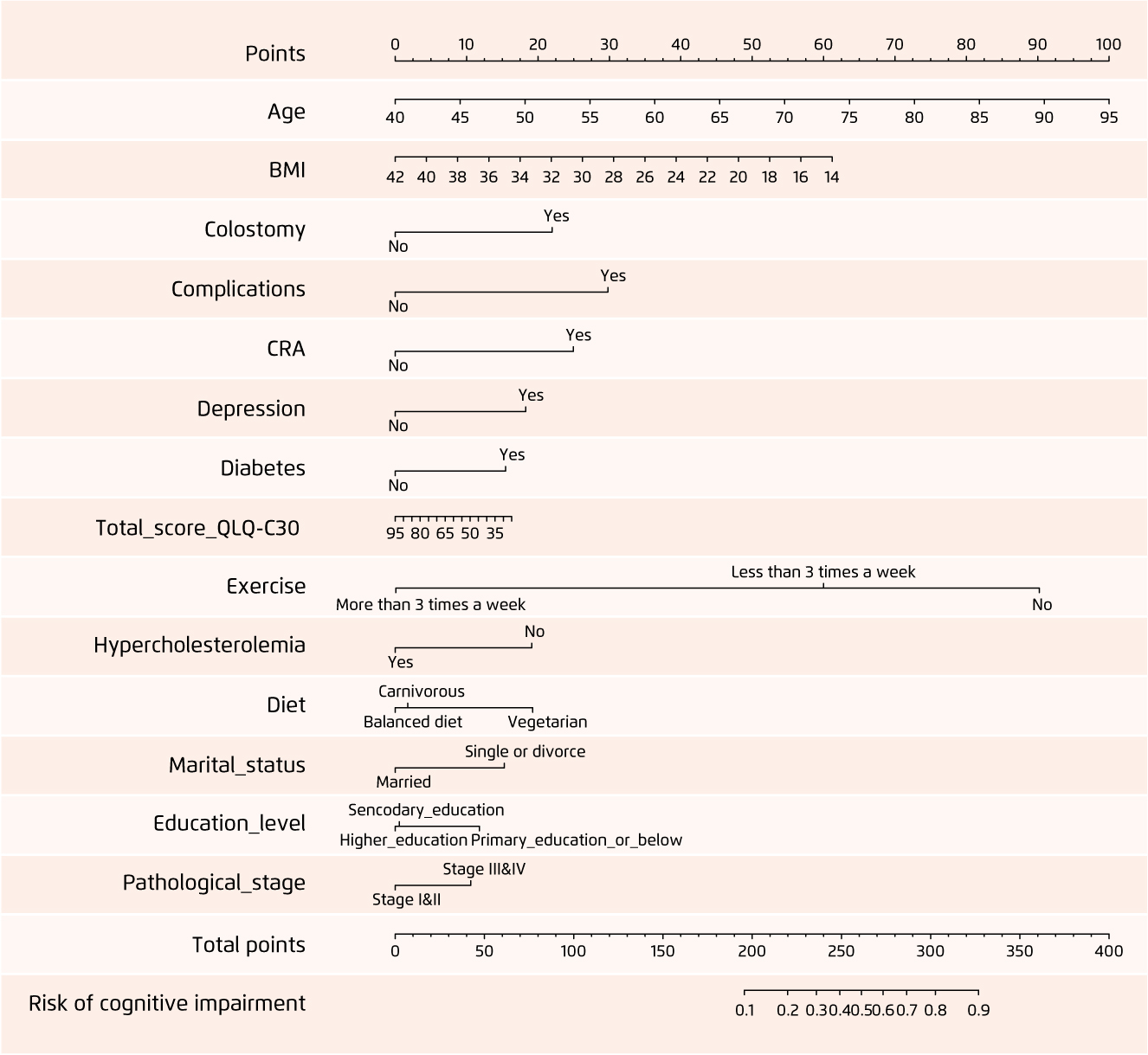
Figure 1.
Visual nomogram prediction model for CRCI risk in colorectal cancer patients post-chemotherapy11.
CRCI: chemotherapy-related cognitive impairment
References:
1. Kim K, et al. Preprints 2021, 2021060330 (doi: 10.20944/preprints202106.0330.v1). 2. Lange M, et al. Ann Oncol. 2019;30:1925-1940. 3. Hong Kong Cancer Registry. Hospital Authority. 10 most common cancers in Hong Kong in 2019. Available at: https://www3.ha.org.hk/cancereg/. Accessed November 2, 2021. 4. The Standard. Lung cancer overtakes colorectal as most common cancer. Available from: https://www.thestandard.com.hk/breaking-news/section/4/181093/Lung-cancer-overtakes-colorectal-as-most-common-cancer. Accessed November 2, 2021. 5. Kuipers EJ, et al. Nat Rev Dis Primers. 2015;15065:1-25. 6. Neugut AI, et al. Clin Colorectal Cancer. 2019;18:133-140. 7. Hwang SY, et al. Cancer Res Treat. 2021;53:1134-1147. 8. Staat K, et al. CJON 2005;9:713-721. 9. Simó M, et al. Neurosci Biobehav Rev. 2013;37:1311-1321. 10. Shaffer VA, et al. Med Care. 2012; 50:849-855. 11. Zhou SP, et al. Biomed Res Int. 2021;2021:6666453.





