Hepatitis C virus (HCV) infection is a global health burden. It affects approximately 0.3-0.5%1 of the local population and is one of the leading causes of liver cancer and a major disease indication for liver transplantation2. Given the established efficacy and safety profile of direct-acting antiviral (DAA) therapies, the World Health Organization (WHO) has set the goal of eliminating hepatitis as a major public health threat by 20303. The pan-genotypic co-formulation of sofosbuvir and velpatasvir (SOF/VEL) yielded an overall cure rate of 98% in both treatment-naïve and treatment-experienced HCV patients, including those with compensated cirrhosis4,5. Essentially, the regimen requires no pre-treatment genotyping and its dosing is simple. These features facilitate the implementation of minimal monitoring approach in HCV management, which aims to enhance patients¡¦ compliance and reduce the burden on healthcare infrastructure.
Clinical Significance of Simplified Monitoring Approach
Although the introduction of DAAs has substantially changed the paradigm of HCV management, the associated cost limits the scale-up of its application. For instance, it has been reported that the costs associated with the recommended diagnostics, such as pre-treatment genotyping and on-treatment monitoring, can be higher than the cost of HCV medications in low-income and middle-income countries6,7. Besides, the overburdened healthcare infrastructure hinders patients’ access to HCV treatment8. Hence, HCV therapy with fewer requirements on pre-treatment diagnosis and on-treatment monitoring would encourage patients’ access to HCV treatment and relieve the burden on healthcare infrastructure.
Petersen et al (2016) reported that a lower daily pill burden would likely enhance adherence9. Besides, given the action of DAAs involves common metabolic pathways, drug-drug interactions (DDIs) with other medications may be a concern10. Therefore, HCV therapy with simple dosing and a safe DDI profile would be preferred.
The issues above thus imply that implementing a minimal monitoring approach, which requires no pre-treatment genotyping and minimal follow-up, with appropriate HCV treatment would help improve patients’ access and adherence to therapy while relieving the burden of healthcare infrastructure.
Minimal Monitoring Approach in HCV Management
In the phase-4 MINMON trial, Solomon et al (2022) evaluated the outcomes of the minimal monitoring approach in HCV management. The approach was characterised by 4 components, including no pre-treatment genotyping, dispensing the entire treatment course (totally 84 tablets) at entry, no scheduled visits or laboratory monitoring, and 2 time points of remote contact (week 4 for adherence and week 22 for scheduling outcome assessment at week 24)11.
399 adult HCV patients from 38 sites initiated single-tablet once-daily oral SOF/VEL treatment for 12 weeks with or without food. The participants could contact clinic staff with their preferred methods, such as email or SMS, in case of any questions or concerns related to the trial or medication. The primary efficacy outcome was sustained virological response (SVR), whereas the primary safety outcome was serious adverse events (AEs)11.
At the SVR assessment, 2 patients were lost to follow-up, and 89% of the 397 participants reported taking 100% of the SOF/VEL treatment during the 12-week treatment period. 95% of the participants who initiated treatment had an SVR. In view of safety outcomes, 4% of participants reported serious AEs between treatment initiation and week 28, but none were treatment-related. No treatment-related unplanned visit, discontinuation or death was observed11.
The results of MINMON trial revealed that SOF/VEL treatment is a safe and effective therapy for generating sustainable SVR in HCV patients. Essentially, the simplified monitoring approach with SOF/VEL regimen effectively reduces the burden on the healthcare system without compromising patients’ compliance. The real-world evidence thus highlights the critical role of the SOF/VEL regimen in making the minimal monitoring approach possible.
SOF/VEL Reduces Risks of DDIs and Minimises Healthcare Burden
as DAAs share pharmacokinetic pathways with many medications commonly administered to the patients10. To investigate the risk of multiple DDIs among HCV patients and the impacts of DDIs on the safety and effectiveness of commonly used DAAs, Turnes et al (2021) conducted a retrospective observational study involving 1.8 million people from a Spanish database12.
1,620 HCV patients treated with either SOF/VEL (n = 730) or GLE/PIB (n = 890) were included in the study. 77.5% of the patients received at least 2 co-medications, while 3 medications were prescribed to each patient on average. Although the number of prescribed co-medication was significantly higher for SOF/VEL group (3.8 vs 2.3, p <0.001), the potential DDIs in this group were lower across the 3 most common co-medication groups, i.e. alimentary, cardiovascular, and nervous system, as compared to GLE/PIB12.
The results demonstrated significantly higher proportions of DDIs in GLE/PIB group with nervous system (8.4% vs. 4.7%,p = 0.002) and cardiovascular (35.0% vs. 10.8%, p <0.001) co-medications12. The results also indicated that the interactions with cardiovascular co-medications were likely to be contraindicated in the GLE/PIB group (9.4%). Importantly, the risk of AEs was significantly higher (p <0.05) in GLE/PIB group as compared to SOF/VEL12. Hence, the risks of multi-DDIs and AEs were less with SOF/VEL compared with GLE/PIB.
Translating the lower risk of DDIs and AEs into clinical benefits, fewer actions, including discontinuation of treatment and dose reduction, were taken by SOF/VEL-treated patients during DAA treatment as compared to GLE/PIB12. DDIs led to more clinic visits in GLE/PIB-treated patients co-administered with antipsychotics and lipid-lowering drugs as compared to SOF/VEL (Figure 1)12. The results thus reflected that SOF/VEL regimen helps reduce the burden on healthcare system.
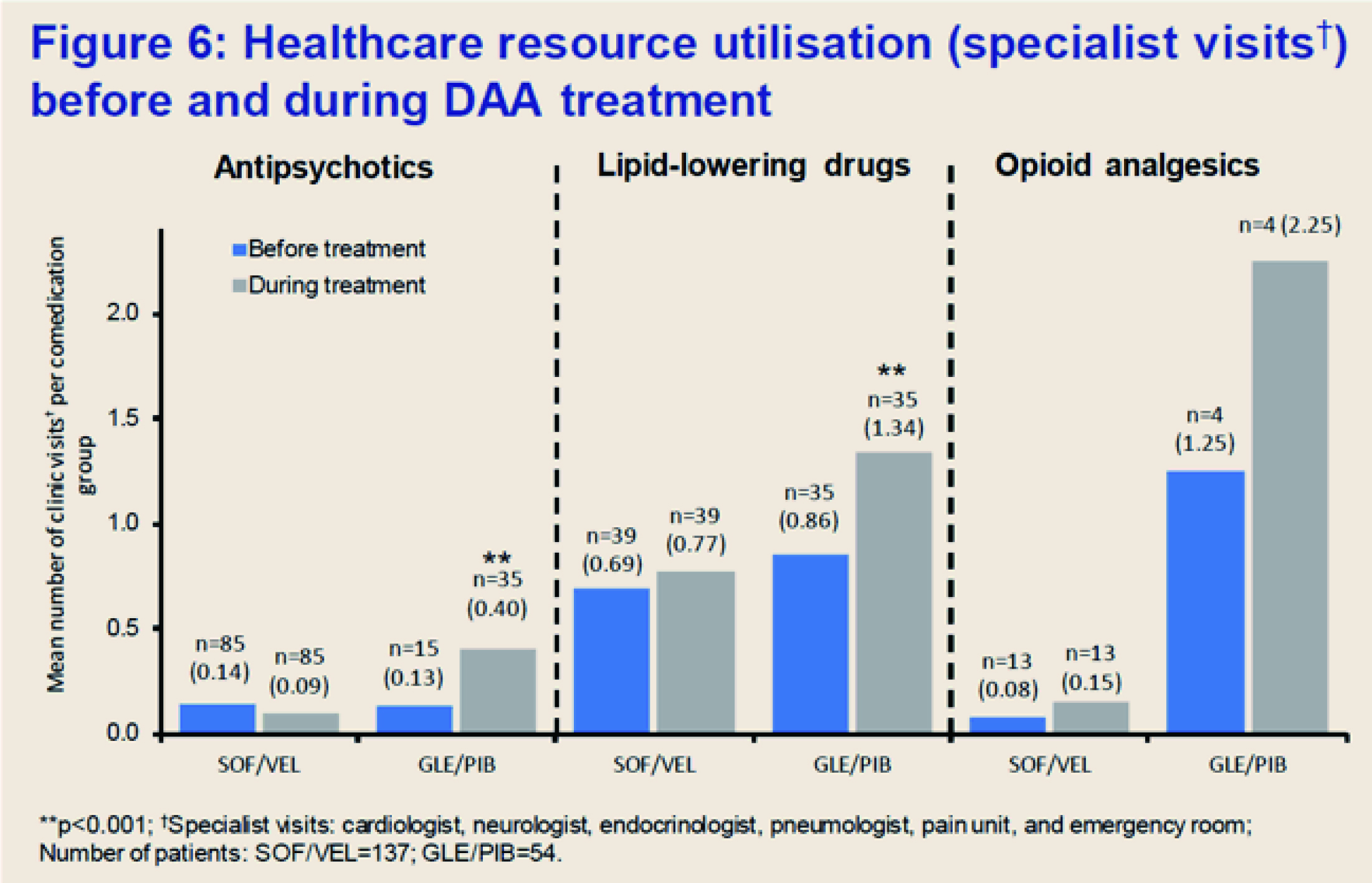
Figure 1. Less DDIs of SOF/VEL led to less healthcare resource utlisation (specialist visits)12
Essential Attributes for HCV Treatments
While efficacy, safety profile, and tolerability are determining attributes for an ideal HCV therapy, simple dosing is crucial in optimising patients’ adherence to treatment. For instance, a recent survey by Andreoni et al (2021) evaluated patients’ and clinicians’ preferences for HCV treatment. The survey included patients from hospitals (n = 372) and drug addiction services (n = 131), as well as clinicians working in the healthcare facilities (hospital = 190, drug addiction services = 142). The relative importance of attributes for the choice of HCV management, including the number of pills per day, duration of treatment and side effects, were evaluated by the participants13.
The results showed the differences in preferences toward HCV treatment between patients and clinicians. Also, participants from hospitals showed different attributes toward treatment characteristics from the drug addiction services counterpart. Notably, while the risk of side effects was an essential attribute for both clinicians and patients in choosing HCV treatment, the relative importance of the number of pills per day ranked higher than that of treatment duration, especially for participants from drug addiction services (Figure 2A and 2B)13. The survey findings implied that more attributes toward therapy characteristics have to be considered in order to select the optimal DAA in HCV management.
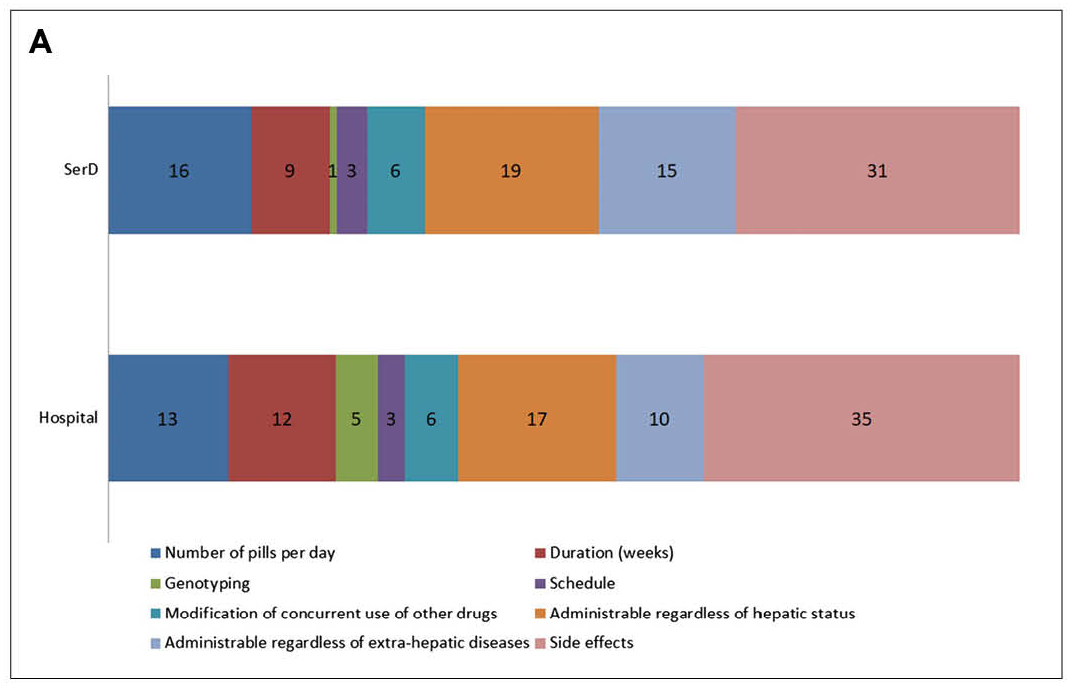
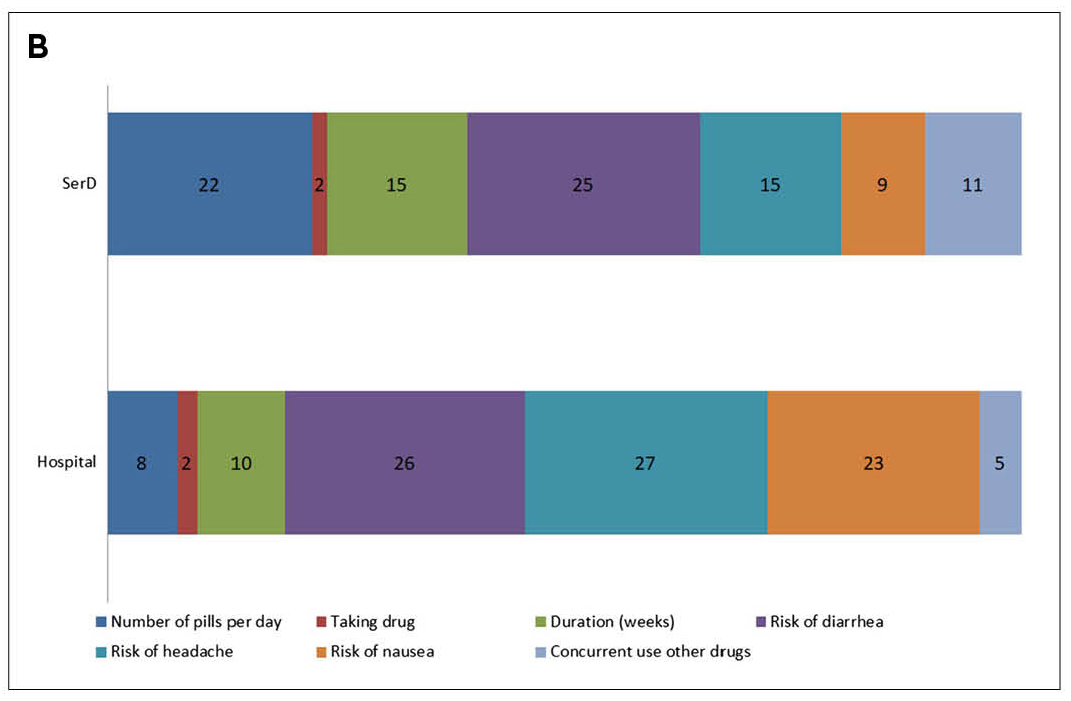
Figure 2. Relative importance (%) of investigated attributes for the choice of treatment for HCV according to A) clinicians and B) patients13, SerD: drug addiction services
The Way towards HCV Elimination
While the efficacy of DAAs in controlling HCV has been well-established, scaling up their application among HCV patients is a significant obstacle to be overcome. Clinical data confirmed that a simplified approach with minimal monitoring effectively controlled HCV in a diverse population. The promising efficacy, simple dosing and safety profile of SOF/VEL regimen would encourage patients’ adherence, whereas its low burden on pre-treatment diagnosis and clinical monitoring would facilitate the implementation of the minimal monitoring approach in clinical settings. Thus, the SOF/VEL therapy is expected to play a crucial role in achieving the WHO’s goal of HCV elimination.
References
1. Liu et al. J Infect Dis 2019; 219: 1924-V33. 2. Lo et al. Hong Kong Med J 2002; 8: 240-V4. 3. Samarasekera et al. Lancet Gastroenterol Hepatol 2021; 6: 611. 4. Feld et al. N Engl J Med 2015; 373: 2599-V607. 5. Curry et al. N Engl J Med 2015; 373: 2618-V28. 6. World Health Organization.Accelerating access to hepatitis C diagnostics and treatment: overcoming barriers in low- and middle-income countries: global progress report 2020. 7. Chaillon et al. PLoS One 2019; 14: e0217964. 8. Kwong et al. J Int Assoc Provid AIDS Care 2015; 14: 112-V5. 9. Petersen et al. Hepatol Int 2016; 10: 310-V9. 10. Gao et al. Int J Gen Med 2021; 14: 289-V301. 11. Solomon et al. Lancet Gastroenterol Hepatol 2022; 7: 307-V17. 12. Turnes. AASLD 2021. 13. Andreoni et al. BMC Infect Dis 2022; 22. DOI:10.1186/S12879-021-06983-Y.





