Colorectal cancer (CRC) is the third most common type of cancer and second most common cause of cancer-related deaths globally1. There were over 1.9 million new cases of CRC globally in 2020 with medium to high human development index (HDI) countries accounting for significant mortality burden2. Why such a difference? Well, the western diet, obesity, sedentary lifestyle, red meat consumption, alcohol and tobacco use among these countries are considered as the risk factors behind the growth of CRC (Figure 1)3. Nonetheless, the incidence of new cases and mortality had been steadily declining over the last few years, except among young (≤50 years), possible due to an increase in CRC screening and better therapy modalities in those above the age of 504. Around 5% of all the CRC cases are attributed to inherited syndromes such as Familial Adenomatous Polyposis (FAP) and Lynch syndrome, also known as hereditary non-polyposis colorectal cancer (HNPCC)5.
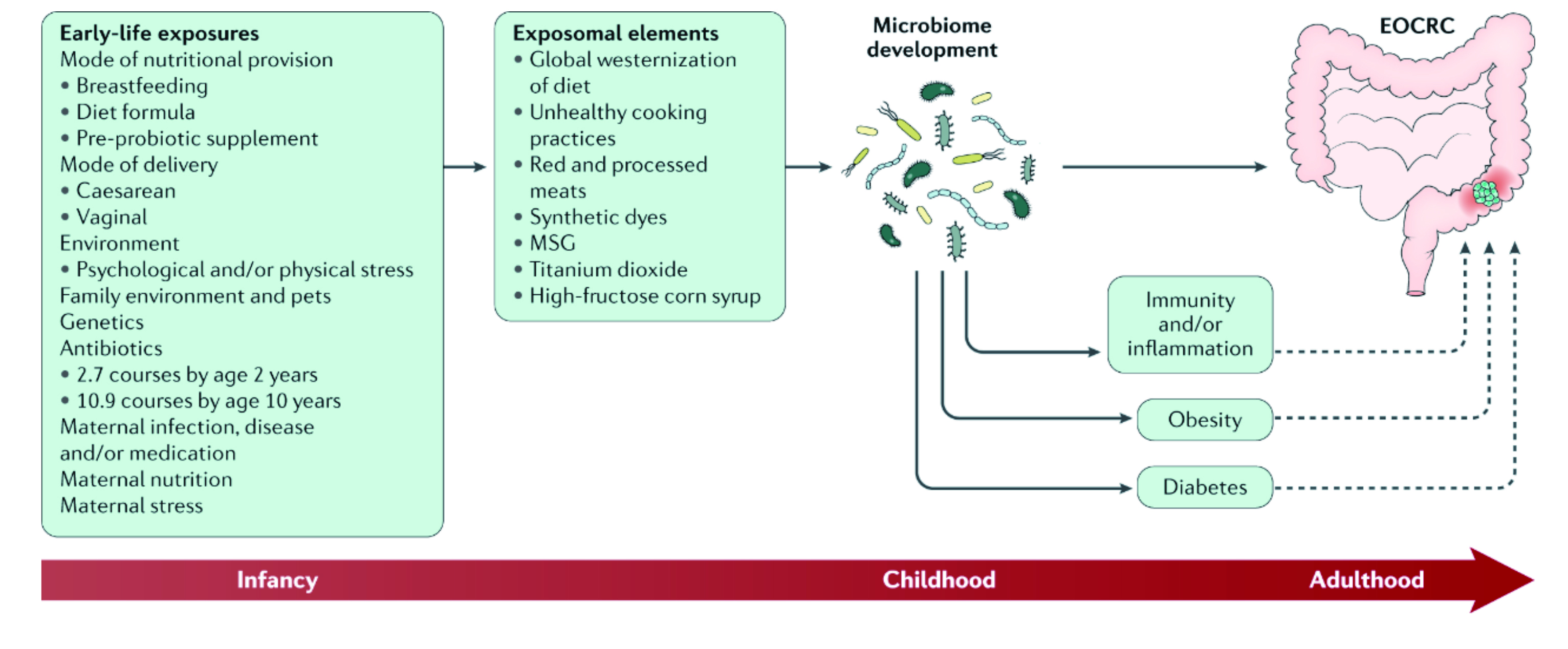
Figure 1. Modulating of gut microbiomes through early-life exposures and exposomal elements may play a role in the development of colorectal cancer (CRC)10.
Furthermore, personal or family history of CRC, adenomatous polyps, and polyps with villous or tubulovillous dysplasia are believed to confer the risk of primary CRC by up to 5% within 5 years4. In addition, medical conditions such as Crohn's disease (CD) and ulcerative colitis (UC) are known to be associated with CRC with estimate incidence of 0.5% annually6. Exposure to radiation, diabetes mellitus, long-term immunosuppression are also considered as the driving force behind CRC4. Very recent epidemiologic studies have identified a positive correlation between cholecystectomy and CRC risk, particularly within the first 6 months after cholecystectomy but these findings are yet to be substantiated7,8. Currently, there are several CRC screening program and initial evaluation often includes barium enema or CT colonography, but ultimately a colonoscopy is required for tissue biopsy (Figure 2)9. Traditionally CRC had mostly been diagnosed in adults over the age of 50 but according to new epidemiological studies, we are now seeing a paradigm shift in CRC among patients under the age of 50 years in European population with more advance staged tumours.
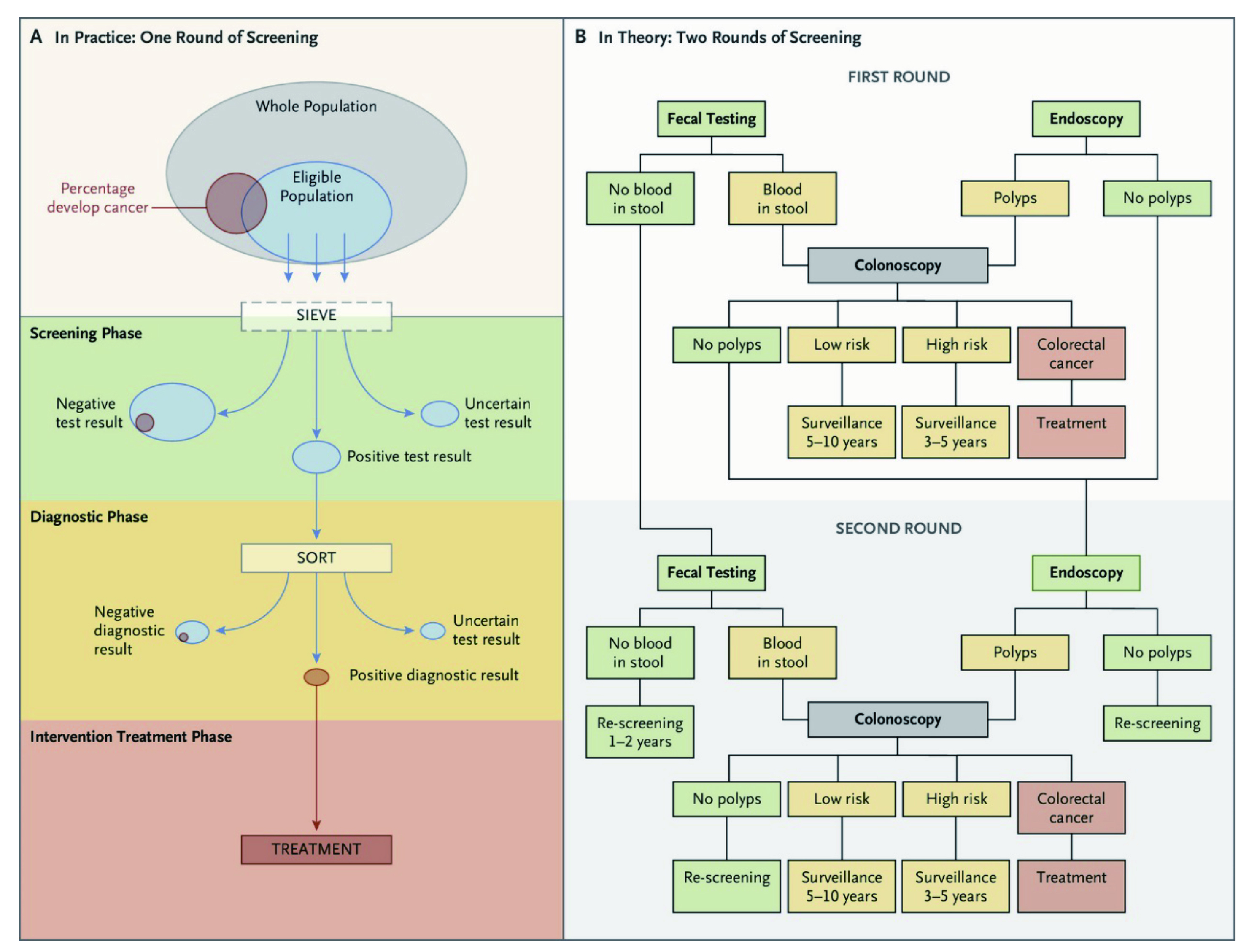
Figure 2. The colorectal cancer screening process where there are two rounds of screening with faecal tests and endoscopic tests26.
CRC in adults below the age of 50 is known as young-onset or early-onset CRC11. But what is the cause and why? The early-onset of CRC is alarming since this subset of CRC patients are not captured by routine CRC screening since the conventional recommendation of CRC screening starts at the age of 5011. Furthermore, younger CRC patients often present with more advanced stages of CRC, highlighting the problem related to delay in diagnosis with possible multifactorial aetiology. According to long-term epidemiological data, the average age of CRC diagnosis decreased from the ages of 67 years to 61.2 years from 1992 to 2017 in the United States (U.S)12. Of note, over a fifth of CRC patients are diagnosed with distance metastatic CRC at lower age compared to having a localised disease at diagnosis12. The early-onset of CRC in young was recently bought to attention by the death of actor, Chadwick Boseman, 43, who was diagnosed with stage III colon cancer in 2016 which eventually progressed to stage IV before 202013.
We may then ask whether there is a missing link between the young and CRC prevalence? If there is, what is it? According research, the risk factors may contribute but does not fully explain the trend14. Some went as far as to hypothesise the change in gut microbiome ecosystem being the missing link between the two, however, this link has not been substantiated15. The precise driving shift towards later-stage diagnosis of CRC among young remains unclear, but some suggest younger patients tend to be diagnosed at later stages partly because doctors may often overlook the early symptoms of CRC such as abdominal pain, blood in the stool, and unintended weight loss for something else in this age group16. Recently, the American Cancer Society (ACS) and a panel backed by the U.S. government have decided to lower their recommended threshold for CRC screening to the age of 45 from initial age of 5011.
There are significant racial disparities among CRC cases with highest morality rates attributed by CRC seen among the American Indian/Alaska Native and Black communities17. Of interest, the reported death rates were 46% higher in American Indian/Alaska Native Men and 44% higher in Black males compared to White counterparts. Why is this the case? Some suggest these disparities in CRC among different ethnic groups may partial be blamed on the approach individuals take when they experience unusual symptoms. In fact, Black males were found to be more inclined to attend and complete CRC screening programme due to their perception of seeing themselves as being strong18.
Furthermore, Whites were the only racial group with a consistent increase in incidence of CRC across all younger ages, with the steepest rise seen after the year 2012. Moreover, a greater increase in the early-onset CRC incidence was observed among males, with more left-sided tumours, with regional and distance spread19. What is the significance of this shift? CRC sidedness is recognised as a prognostic factor for survival with left-sided lesions often having a better outcome than the right-sided lesions. In addition, recent studies have reported obesity and racial disparity plays a significant role in long-term outcomes of CRC. For instance, obese Black patients with CRC often have right-sided CRC compared to the left, hence poorer outcome compared to the White and Hispanics20,21. It raises more questions than answers on what can be done and whether something environmental may be promoting more CRC.
The recent rise of young individuals under the age of 50 with CRC is a startling trend in need of greater focus and research. The risk factor modification and screening are the primarily the largest contributors to the overall decline in CRC mortality among those above the age of 5022. Moreover, the incentives by the ACS to reduce the screening age to 45 years may help ameliorate the increasing burden of young-onset CRC23. Some may then ask, is it cost effective to screen everyone below the age of 50? The answer to this is partly, mainly because screening at younger age increases the risk of false positive, which also means initiation colonoscopy screening early, putting patient at risk of complications related to colonoscopies, in addition to increasing the cost by up to 17% to achieve a merely increase in life-years gained by 6.2%24,25.
Therefore, CRC screening should occur before the age of 50 for individuals at elevated risk for CRC, particularly those with family history of hereditary CRC syndromes and inflammatory bowel disease (IBD)27. Nevertheless, the response to CRC screening remains low among adults aged 50 to 54 years with only 47.6% attended CRC screening programmes in 2018 compared to 78% of adults aged 70-7528. Therefore, apart from CRC screening, educating primary care providers and the larger population on the increasing incidence and characteristic symptoms is equally important29. In conclusion, healthcare providers should stay vigilant on this changing trend and help tackle the alarming rate of CRC through coordinated effort with their patients early.
References
1. Sawicki T, et al. Cancers (Basel) 2021; 13(9). 2. Arnold M, et al. Gut 2017; 66(4): 683-91. 3. Wong MCS, et al. Clin Gastroenterol Hepatol 2021; 19(5): 955-66.e61. 4. Lotfollahzadeh S, et al. Treasure Island (FL): StatPearls Publishing 5. Allen J, et al. Genome Med 2019; 11(1): 11. 6. Olén O, et al. Lancet 2020; 395(10218): 123-31. 7. Lee J, et al. J Prev Med Public Health 2018; 51(6): 281-8. 8. Zhang Y, et al. PLoS One 2017; 12(8): e0181852. 9. Wills B, et al. Curr Probl Cancer 2018; 42(6): 593-600. 10. Hofseth LJ, et al. Nature Reviews Gastroenterology & Hepatology 2020; 17(6): 352-64. 11. Done JZ, et al. World J Gastrointest Oncol 2021; 13(8): 856-66. 12. Liu A, et al. Int J Colorectal Dis 2023; 38(1): 45. 13. Kahlam A, et al. Cureus 2022; 14(5): e24959. 14. Venugopal A, et al. Curr Treat Options Gastroenterol 2019; 17(1): 89-98. 15. Rebersek M. et al. BMC Cancer 2021; 21(1): 1325. 16. Siegel RL, et al. Am Soc Clin Oncol Educ Book 2020; 40: 1-14. 17. Pankratz VS, et al. Cancer Epidemiol 2022; 80: 102229. 18. Rogers CR, et al. Int J Environ Res Public Health 2022; 19(5). 19. Chang SH, et al. Clin Gastroenterol Hepatol 2022; 20(6): e1365-e77. 20. Kamath SD, et al. Cancer Med 2021; 10(21): 7542-50. 21. Ulanja MB, et al. J Surg Oncol 2023; 127(1): 109-18. 22. Edwards BK, et al. Cancer 2010; 116(3): 544-73. 23. You YN, et al. JCO Oncol Pract 2020; 16(1): 19-27. 24. Peterse EFP, et al. Cancer 2018; 124(14): 2964-73. 25. Wolf AMD, et al. CA Cancer J Clin 2018; 68(4): 250-81. 26. Helsingen Lise M, et al. NEJM Evidence 2022; 1(1): EVIDra2100035. 27. Gupta S, et al. J Natl Compr Canc Netw 2017; 15(12): 1465-75. 28. Liu PH, et al. Cancer Epidemiol Biomarkers Prev 2022; 31(9): 1701-9. 29. Hossain MS, et al. Cancers (Basel) 2022; 14(7).





