
Gestational Diabetes Mellitus: The Challenges and Management During and After Pregnancy
Gestational diabetes mellitus (GDM) is a serious pregnancy complication, and according to the International Diabetes Federation (IDF), the disease affects approximately 14% of pregnancies worldwide, representing around 18 million births annually1. GDM has important health implications associated with long-term health problems for both, the mother and the child. The mother has a greater possibility of undergoing a caesarean section, experiencing pre-eclampsia and developing type 2 diabetes mellitus (T2DM). On the other hand, the baby can be diagnosed with foetal macrosomia, shoulder dystocia and other physiological and metabolic abnormalities such as neonatal hypoglycaemia, which can further progress to obesity with insulin resistance in young adulthood2. Diagnosing and treating GDM can subsequently reduce perinatal complications and reduce the risk of children developing obesity. Lifestyle modifications and interventions that mitigate obesity are the primary approach in preventing or delaying diabetes3.


Table 1. Risk factors associated with GDM4
The Aetiology of GDM
Impaired beta-cell function and insulin sensitivity are either directly or indirectly associated with these risk factors that consistently emerge (Table 1)4. As a result of reduced insulin sensitivity, hyperglycaemia occurs in patients with gestational diabetes mellitus (GDM) as opposed to patients undergoing a normal pregnancy (Figure 1)4. Beta-cells are overburdened and in response, additional insulin is produced. The direct contribution of glucose to beta-cell failure is described as glucotoxicity. Hence, once dysfunction of beta-cells begin, a vicious cycle of hyperglycaemia, insulin resistance, and further beta-cell dysfunction is set in motion4. Other evidence contributing to the aetiology of GDM include adipose expandability, low-grade chronic inflammation, gluconeogenesis and oxidative stress – all of which require further understanding4.
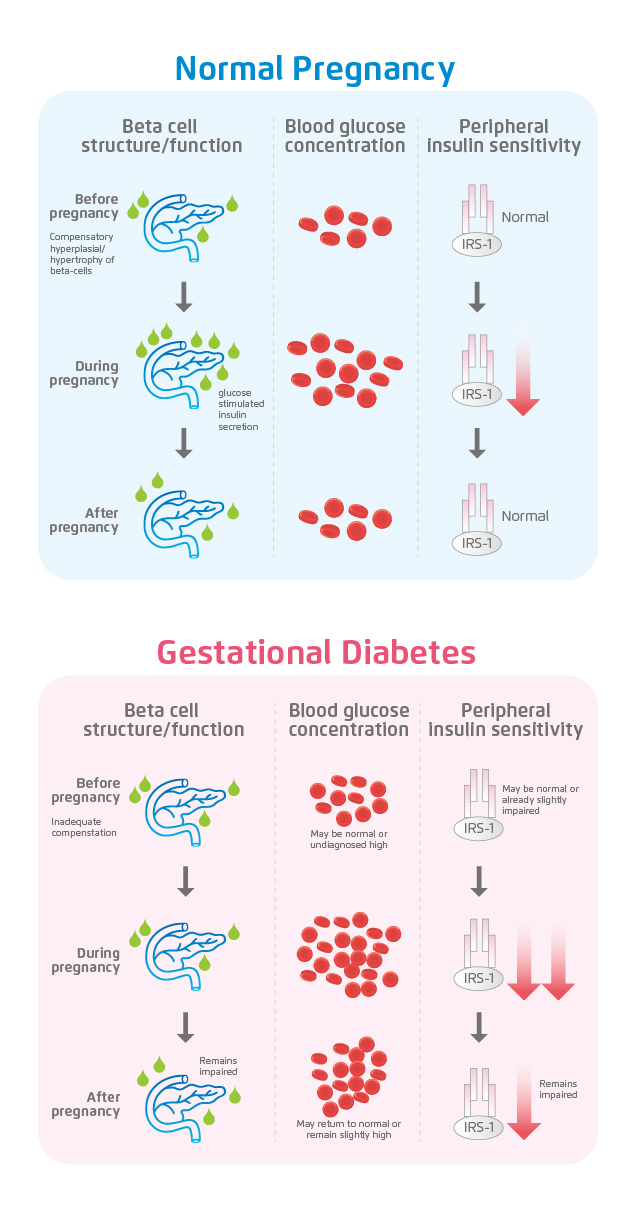
Figure 1. The relationship between β-cell dysfunction, insulin resistance and GDM4
Complications during Pregnancy and Birth
GDM is associated with an increased risk of hypertensive disorders such as gestational hypertension, preeclampsia and eclampsia5. The Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study, which included 21,364 women, examined the association of fasting C-peptide, body mass index (BMI) and maternal glucose with risk of preeclampsia. Results showed that the level of glucose during the first glucose tolerance test was positively correlated with the risk of preeclampsia. Moreover, a direct correlation between the caesarean section rate and maternal glycaemia with an overall frequency of 23.7% was also found in the same study6.
Additionally, it was derived in another study, the International Association of Diabetes and Pregnancy Study Groups (IADPSG), that the percentage of body fat in new-borns, maternal glycaemia and foetal insulin levels estimated by cord C-peptide level were strongly positively correlated7. Shoulder dystocia can be a serious birth complication and when the birth weight exceeds 4 kg, it has been shown that there is a clear association between increased foetal size and the risk of shoulder dystocia5. Although extremely rare today, in more extensive cases, older studies have shown that the risk of stillbirth can also be increased fourfold8.
Long-term Consequences of GDM for the Mother and Offspring
GDM is not only associated with adverse pregnancy outcomes but can also significantly increase the risk for long-term consequences in both the mother and their offspring5.
Even though majority of women return to a euglycaemic state soon after delivery, women who have had GDM have an increased risk for developing type 2 diabetes mellitus (T2DM)5. Approximately 60% of women with a prior history of GDM develop T2DM later in life and with each additional pregnancy, the risk of developing T2DM is increased by threefold4. Risk factors including central obesity, insulin resistance, hypertension and dyslipidaemia are considered to be associated with the development of T2DM as well as cardiovascular diseases (CVD). The risk of CVD is comparatively 70% higher in women with previous GDM as opposed to women having normoglycaemic pregnancies5.
GDM not only poses long-term consequences for the mother but also for the infant. The foetus’ endogenous production of insulin and insulin-like growth factor 1 (IGF-1) is stimulated with an increase in the placental transport of glucose, amino acids and fatty acids, resulting in foetal overgrowth4. As a result, GDM is associated with incident diabetes in offspring during childhood and adolescence9. There is almost double the risk of developing obesity, T2DM, CVD and associated metabolic diseases in babies that are born of GDM pregnancies. Glucose tolerance can be impaired and detected as young as five years old. Therefore, females of GDM pregnancies are more likely to experience GDM in their own pregnancies, conferring to an intergenerational cycle of GDM4.
Effective Prevention Strategies
In younger adults, the diagnosis of GDM can be a unique opportunity for prevention of future diabetes10. However, intervention with lifestyle modifications in previous large population studies are either absent or have yielded conflicting results2. Moreover, lifestyle intervention performed during pregnancy has shown further inconsistency in the prevention of GDM. Lifestyle interventions include individualised counselling on diet, physical activity and weight control. It was believed in a study conducted last year that the intervention initiated before pregnancy would be more beneficial for the prevention of GDM in high-risk women11. Although a lifestyle intervention initiated during first trimester of pregnancy showed a 36% reduction in the incidence of GDM12, the same type of intervention initiated before conception had no effect on GDM incidence.
Moreover, certain potential therapeutics for GDM can also be highlighted – there is growing evidence showing that myo-inositol combined with an appropriate therapeutic regimen for GDM, can potentially provide additional benefits to pregnant women with GDM13. Additionally, probiotics have also shown a reduction in fasting blood glucose, inflammation and oxidative stress14. Another study conducted on GDM mice found that naringenin ameliorated GDM symptoms as well as improved glucose and insulin tolerance, thus resulting in an improved insulin sensitivity15.
Overall, it is strongly speculated that a longer, more frequent and intensive lifestyle intervention in the preconception period, as well as potential therapeutics among a larger study population would have a positive impact on the incidence of GDM and subsequently on perinatal and neonatal outcomes.
References
1. International Diabetes Federation. IDF Diabetes Atlas;8th Edition: Belgium. 2017.2. Agha-Jaffar R, et al. Nat Rev Endocrinol 2016;12:533-546. 3. Buchanan TA, et al. Nat Rev Endocrinol 2012;8:639-649. 4. Plows JF, et al. Int J Mol Sci 2018;19:3342. 5. Kampmann U, et al. World J Diabetes 2015;6:1065-1072. 6. Yogev Y, et al. Am J Obste Gynecol 2010;202:255.e1-255.e7. 7. Metzger BE, et al. Diabetes Care 2010;33:676-682. 8. O’Sullivan JB, et al. Am J Obstet Gynecol 1973;116:901-904. 9. Blotsky AL, et al. CMAJ 2019;191:E410-E417. 10. Goveia P, et al. Front Endocrinol (Lausanne) 2018;9:583. 11. Rönö K, et al. Int J Womens Health 2018;10:493-501. 12. Koivusalo SB, et al. Diabetes Care 2016;39:24-30. 13. Guardo FD, et al. J Complement Integr Med 2019. [Epub ahead of print] 14. Chen Y, et al. Med Clin (Barc) 2019. [Epub ahead of print] 15. Li S, et al. Nutr Diabetes 2019;9:28.





