
Human gut harbours approximately 1014 microorganisms, also known as gut microbiota, which helps to maintain gut ecosystem and the gut-brain axis. Bacteria, particularly Firmicutes and Bacteroidetes are predominant phylum that are extensively studied, and their alteration results in “gut dysbiosis.” Gut dysbiosis induces gut inflammation and diseases. Diet, lifestyle, antibiotics, stress, and disease are among the risk factors of gut dysbiosis. Furthermore, westernised diet has shown to induce gut dysbiosis by altering availability of microbiome-accessible carbohydrates (MACs) and generation of short-chain fatty acids (SCFAs).
Gut dysbiosis is treated through various modalities which include the use of prebiotics, probiotics, in addition to recent emergence use of faecal microbiota transplantation (FMT) in conditions such as Clostridium difficile infection (CDI) and inflammatory bowel disease (IBD). Nonetheless, dietary measures such as increase fiber intake remains important for reversing gut dysbiosis as it facilitates carbohydrates uptake and synthesis of SCFA that help maintain the gut health.
The human gut is colonised by microbes during early life, and helps to shape our immune system, metabolic function, and potentially future health. So, one may ask where does these microbes come from? Thanks to recent animal studies, we now know that maternal microbes are transmitted to offspring during childbirth and this represents a key step in colonisation of the sterile infant gut as in Figure 1 1. There are approximately 1014 microorganisms that resides in the human gastrointestinal tract (GIT), termed “gut microbiota.”
Gut microbiota include bacteria, viruses, fungi, and protozoa with bacteria being extensively studied in literature 3. The two predominant bacterial phylum that resides our GIT includes Firmicutes and Bacteroidetes. Microbes, as we all know are often associated with a wide range of diseases and are often perceived as “bad for health.”
Nonetheless, emerging studies have shown that gut microbiota being an important primary contributor for biosynthesis of vitamins, essential amino acids, in addition to generating important metabolic byproducts which helps maintain the intestinal immunity, gut mucosal barrier, and regulates gut-brain axis 2,3,4. Moreover, the gut microbiota and their diversity in the intestinal tract helps promotes healthy intestinal immunity through activation of toll-like receptors (TLR) expression, antigen presenting cells (APC), and maintain differentiated T-cells and lymphoid follicles populations 1. This symbiotics microbial colonisation in the human intestinal tract is crucial and is maintained through dietary measures.
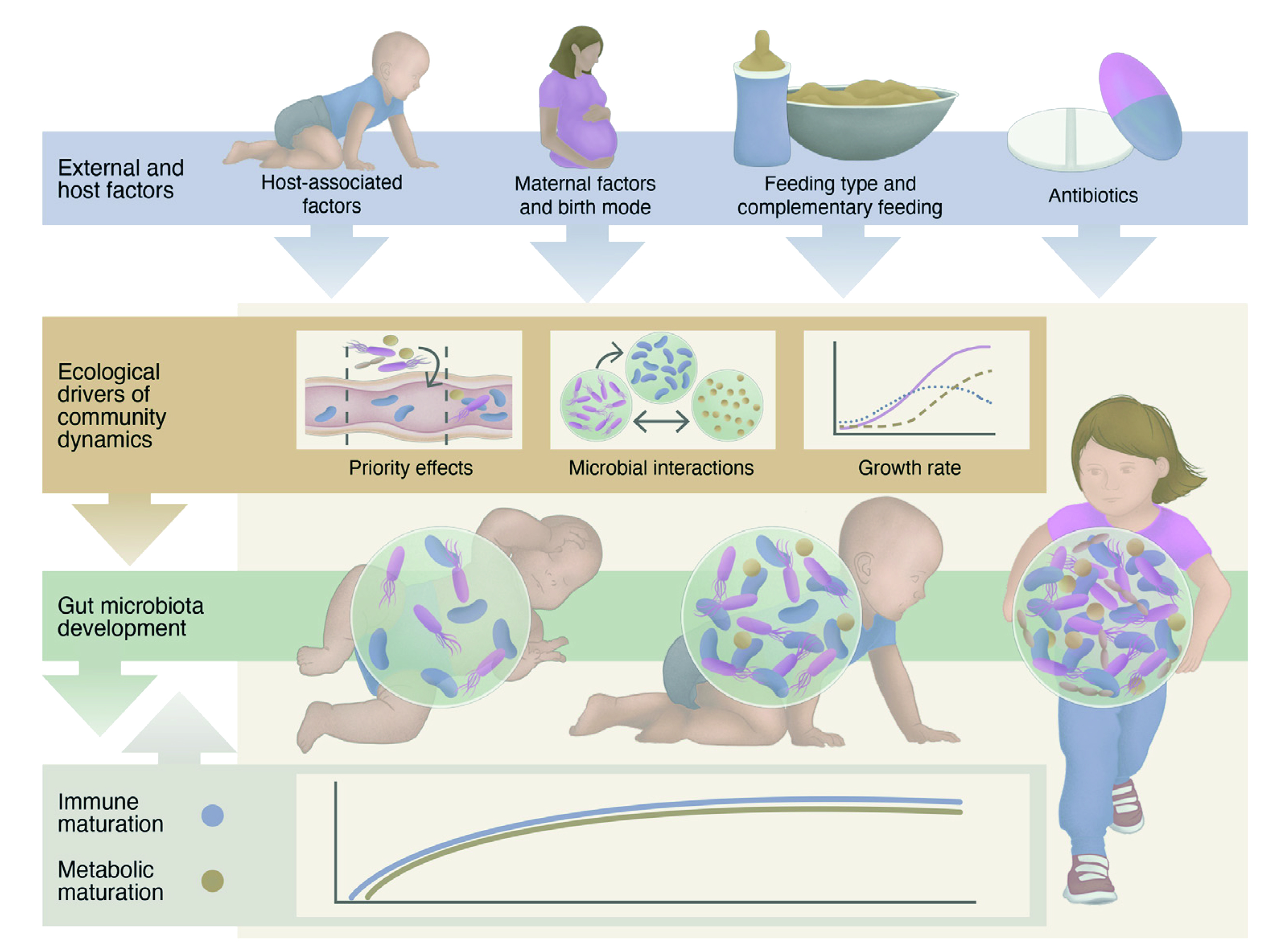
Figure 1. An overview on factors affecting infant’s immunity and gut microbiota development 2
There are several factors which may affect the gut microbiota ecosystem and among these, diet, lifestyle, stress, antibiotics, and diseases contributes significantly to the development of dysbiosis 4. The effects of dietary changes on gut microbiota have been extensively studied and epidemiological data has shown disturbances of colonic microbiota may exacerbate chronic malnutrition and growth retardation in resources deprived nations such as Africa, whereas introduction of westernised diet in other parts of the world promoted obesity and type 2 diabetes mellitus (T2DM) pandemic 5. We now know that diet play an important role in shaping the gut microbiota ecosystem since animal studies have reported dietary alteration may induce a large, temporary gut microbiota shift even in a short time span 6. These changes may then predispose an individual to developing several chronic diseases which includes inflammatory bowel disease (IBD), obesity, T2DM, cardiovascular disease (CVD) and colorectal cancer (CRC) 6. Now the question arises, how does the diet affect the gut microbiota population? To answer this, we need to first understand the early symbiotic relationship between the human gut and microbiota.
During infancy, gut microbiota matures and over the next few years, it undergoes changes as we grow. The transition from infancy to adulthood allows the gut microbiota to co-evolve and interact to maintain the gut homeostasis 8. Furthermore, gut microbiota often require accessible carbohydrates, also known as microbiome-accessible carbohydrates (MACs) which are obtained through vegetables, whole grain foods that we eat and often lacks in processed foods 9. In other words, the food we eat are the main source of fuel for the gut microbiota since microbiotas generates short-chain fatty acids (SCFAs) which are essential for maintaining gut health 10. Therefore, any changes in our diet results in changes in the structure of gut microbiota in our intestinal tract. For instance, fermentation of dietary fibers reduces pH from 6.5 to 5.5 in our colon, thereby slows down the growth of gram-negative microbes such as Salmonella and Escherichia coli 8,11. Furthermore, insufficient dietary fiber intake promotes the growth of mucosal pathogens such as Citrobacter rodentium, thereby, increases host susceptibility of developing diseases such as colitis 12.
Gut microbiota is resilient to short-term dietary changes, but not to long-term changes as these induce changes in bacterial taxa, diversity, and carbohydrate utilisation ability 13. For instance, westernised diets often contain ultra-processed foods with excess fat, sugars, and additives with small amounts of micronutrients. These changes then allow reshaping of gut microbiota, providing the fertile soil for microbes to induce inflammatory diseases and gut dysbiosis 14.
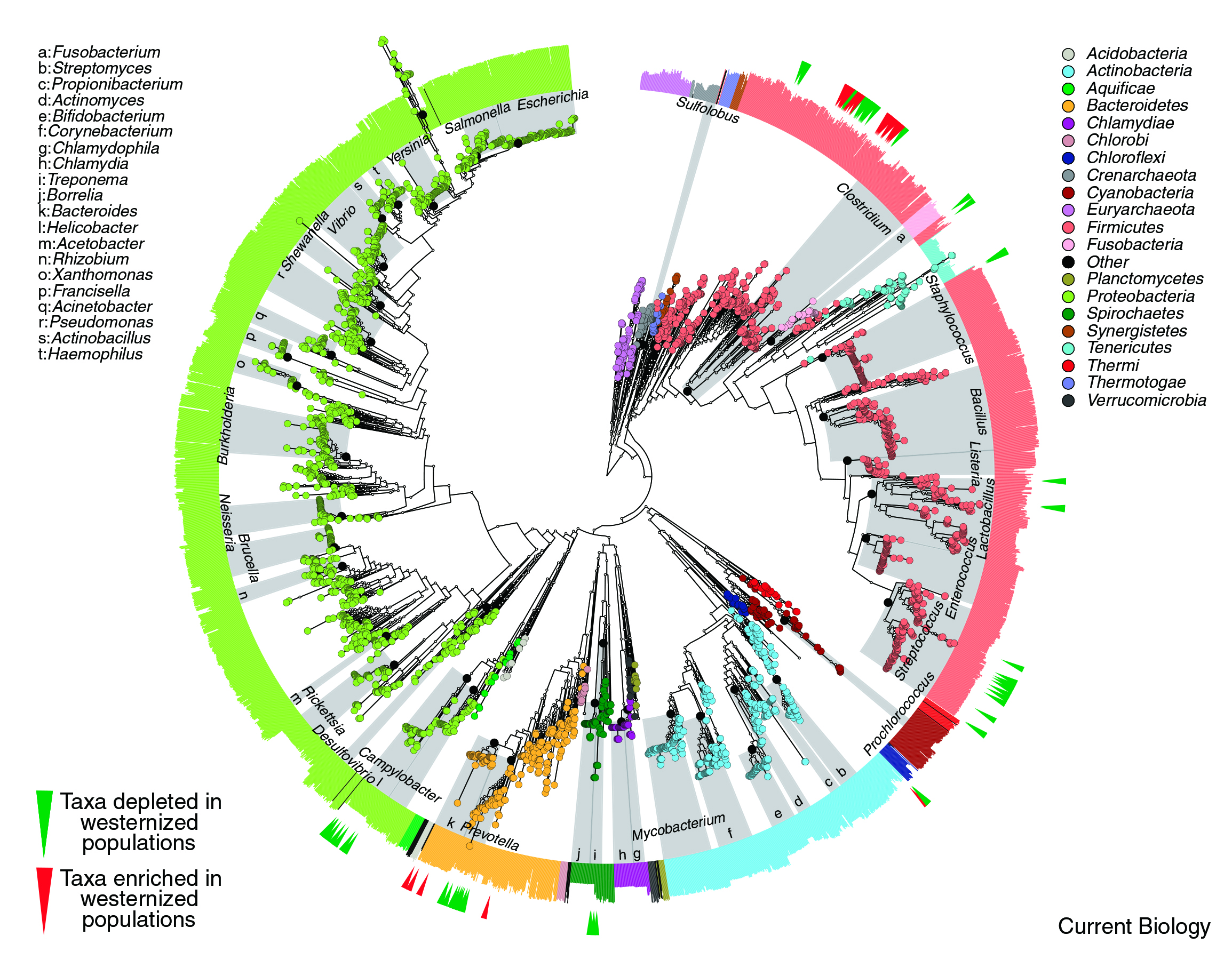
Figure 2. High-resolution of microbial treee phylogenetic extracted from 3,737 reference genome demonstrates a loss of microbiota secondary to westernised diet 7.
Gut homeostasis is closely related to the gut microbiota, and this notion is supported by the emerging evidence on both animal and human studies which suggests local inflammatory conditions such as IBD are related to an increased activity of inflammatory cells such as T-cells as seen in Figure 3. These together with other local factors such as cytokines, tumour necrotic factor (TNF) may affect the gut mucosal membrane transporter, thereby contribute to the hypothesis of “leaky gut” in disease state 15.
Moreover, gut dysbiosis in other systemic disease states such as T2DM is characterised by enriched membrane transport of glucose and branched-chain amino acids with a reduction in essential metabolic products such as butyrate synthesis 16. Similar findings have been seen in obesity with alteration in intestinal Bacteroides: Firmicute ratio, with great abundance of Firmicutes and background inflammation in the gut. These are not an isolate findings, instead studies on mice have shown gut microbiota transplantation from obese to lean mice may transmit obese phenotype and promoting obesity among lean mice 17.
Now the question remains is how is gut dysbiosis related to CRC? Thanks to the ongoing research, we now know that CRC has several risk factors, including IBD, obesity and T2DM. Furthermore, there is a lower population of butyrate-producing bacteria with increase population of pathogenic species such as Akkermansia muciniphila and Fusobacterium nucleatum reported among CRC patients. Both species of bacteria are associated with strong local inflammatory responses, in addition to mediate lymphatic metastatic spread of the tumour 18. Therefore, alteration of gut microbiota may decrease regulation of colonic epithelial proliferation, further promoting pre-carcinogenic properties and neoplasm 19. Similar findings have been reported in CDI where gut microbiota diversity is severely compromised by antibiotics use and loss of colonization of healthy microbes 20.
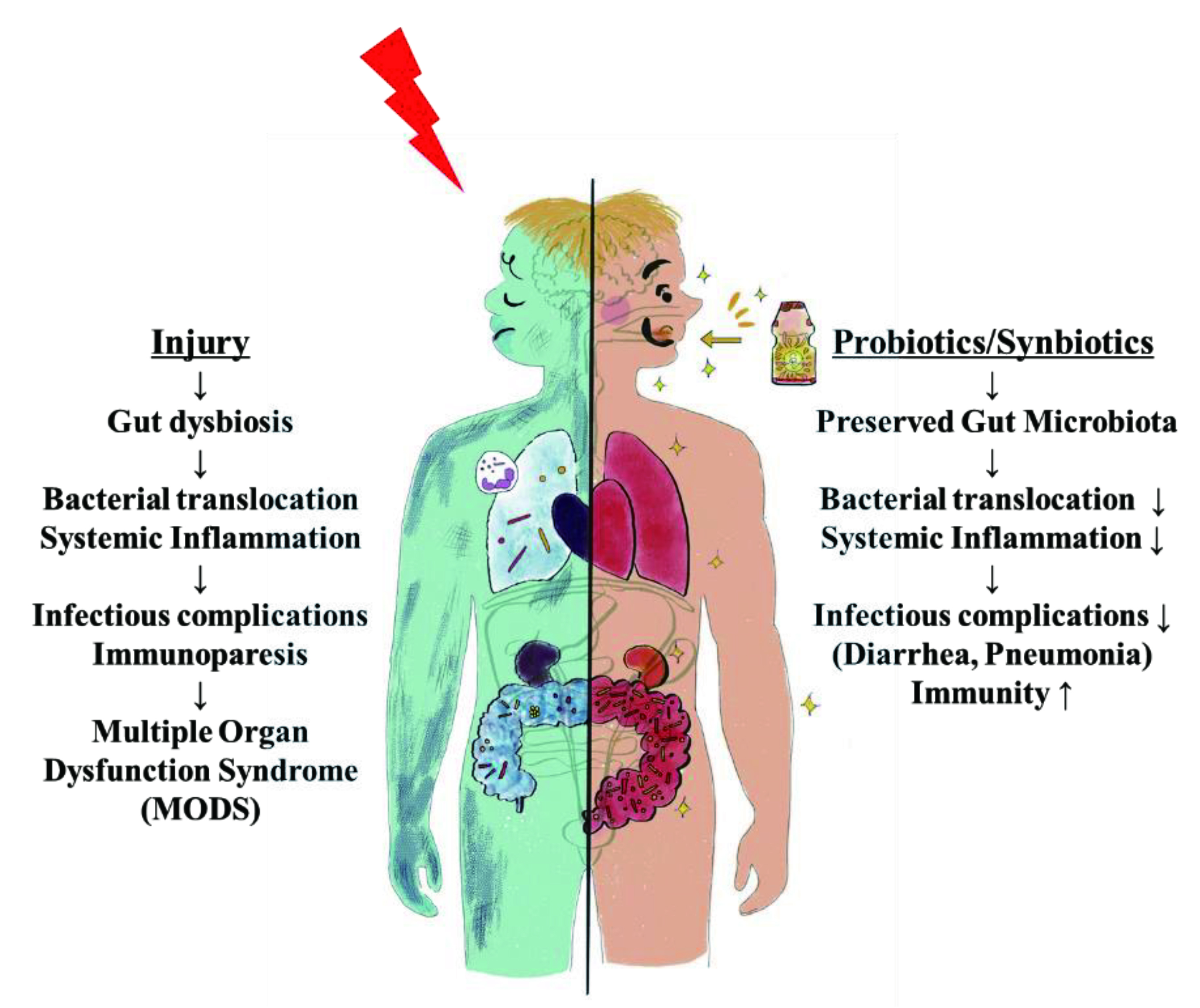
Figure 3. Gut dysbiosis: loss of gut microbiota homeostasis following systemic injury increases gut inflammation affecting multiple organs. Probiotics and synbiotics help maintain gut microbiota, prevent further deterioration and sequelae 21.
There are many therapeutic strategies developed to re-establish the microbiota-gut-brain axis including the use of prebiotics and probiotics 22. The rationale behind the use of prebiotics and probiotics is best explained by their mechanism of action as probiotics displaces pathogenic microbes while prebiotics allow overgrowth of probiotic microbes, thereby promoting the rebalance of microbiota ecosystem in the gut. Emerging studies demonstrated the use of synbiotics, which is a combination of prebiotics and probiotics among surgical patients reduces postoperative infectious complications and ventilator-associated pneumonia 21.
The more recent approach in treating gut dysbiosis included the use of FMT which has been experiment in IBD patients with recurrent Clostridium difficile-induced pseudo-membrane colitis or infections 23,24. The results have been promising with a good safety profile reported. Furthermore, FMT use has also been experimented in obesity and data obtained from a double-blinded randomised controlled trial where 61 obese patients with T2DM were randomly assigned either FMT plus lifestyle intervention, (LSI), FMT alone and sham transplantation. The study concluded those on FMT plus LSI led to a more favourable change, in addition to having an overall improvement in metabolic markers 25. Regardless of the intervention used, composition of dietary intake is important, but why? A diverse diet is associated with a greater microbial alpha-diversity (how diverse a single sample is) as it provides a substrate for different taxa of microbiota within the gut 26.
These findings have been substantiated in an observational longitudinal study where faecal samples obtained from cohorts on a diverse plant-based diet showed a more diverse metagenomic among faecal samples, in addition to sustaining eubiosis 27. In fact, higher intake of dietary fibers can help to improve the abundance of Bifidobacterium genus which normally resides in healthy gut and counteracts against inflammation 28. Moreover, dietary fibers also provide gut microbiota the necessary complex carbohydrates that are required for synthesis of short-chain fatty acids (SCFA) which helps to maintain gut integrity and immunity 28,29. In conclusion, reversal of gut dysbiosis through dietary intervention represents an important step for maintaining gut health in conjunction with other intervention used to restore the gut homeostasis.
References
1. Walker et al. Pediatr Obes 2017; 12 Suppl 1(Suppl 1): 3-17. 2. Jian et al. eBioMedicine 2021; 69: 103475. 3. DeGruttola et al. Inflamm Bowel Dis 2016; 22(5): 1137-50. 4. Madison et al. Curr Opin Behav Sci 2019; 28: 105-10. 5. Wilson et al. J Transl Med 2017; 15(1): 73. 7. Segata. Curr Biol 2015; 25(14): R611-3. 8. Su et al. Frontiers in Nutrition 2021; 8. 9. Jenkins et al. Lancet 1976; 2(7978): 172-4. 10. Koh et al. Cell 2016; 165(6): 1332-45. 11. Holscher. Gut Microbes 2017; 8(2): 172-84. 12. Desai et al. Cell 2016; 167(5): 1339-53.e21. 13. Leeming et al. Nutrients 2019; 11(12). 14. Zinöcker et al. Nutrients 2018; 10(3). 15. Greenblum et al. Proc Natl Acad Sci U S A 2012; 109(2): 594-9. 16. Qin et al. Nature 2012; 490(7418): 55-60. 17. Turnbaugh et al. Nature 2006; 444(7122): 1027-31. 18. Castellarin et al. Genome Res 2012; 22(2): 299-306. 19. Elinav et al. Nat Rev Cancer 2013; 13(11): 759-71. 20. Zhang et al. Anaerobe 2015; 34: 1-7. 21. Shimizu et al. Nutrients 2021; 13(7). 22. Gagliardi et al. Int J Environ Res Public Health 2018; 15(8). 23. Fischer et al. Gut Microbes 2017; 8(3): 289-302. 24. Newman et al. Gut Microbes 2017; 8(3): 303-9. 25. Ng et al. Gut 2022; 71(4):716-23. 26. Amamoto et al. Benef Microbes 2022; 13(6): 453-64. 27. Johnson et al. Cell Host Microbe 2019; 25(6): 789-802.e5. 28. Shen. Nestle Nutr Inst Workshop Ser 2017; 88: 117-26. 29. Blaak et al. Benef Microbes 2020; 11(5):411-55.





