
The global prevalence of diabetes mellitus has reached an alarming level, which was estimated to be 10.5% in 2021 and rising to 12.2% in 2045. The disease contributes to life-threatening, disabling, and costly complications as well as reducing life expectancy1. Thanks to the recent advancement in pharmacotherapy, various therapeutics for type 2 diabetes mellitus (T2D) acting on different mechanisms have been emerging. These therapeutics significantly improve glycaemic outcomes in patients with T2D and yield additional health benefits, such as obesity control2. Nonetheless, recent opinions argued the conventional management of T2D relying on the algorithms for the sequential addition of pharmacotherapies to achieve target glycaemic control might not effectively achieve treatment targets and increase costs. In contrast, the personalised approach for managing T2D, which takes a wide spectrum of factors into account and is tailored to the individual, is advocated to facilitate optimal treatment outcomes.
The Recent Pharmacological Advancement in T2D
T2D is primarily characterised by insulin resistance (IR) and a defect in insulin secretion, which jointly lead to hyperglycaemia. Persistent hyperglycaemia subsequently causes glucotoxicity, increased oxidative stress and lipotoxicity, which causes further reduction in insulin secretion due to progressive beta-cell failure3. As decrease in glycated haemoglobin A1C (HbA1c) is associated with risk reduction for vascular complications and diabetes-related mortality4, reducing HbA1c is the target of most T2D therapeutics.
Insulin therapy is a treatment option when beta-cell function is severely limited, but it appears to be beneficial in newly diagnosed patients with T2D as well. According to their pattern of action, the available preparations of insulin are classified into basal, prandial or combination. Alternatively, insulin preparations can be classified into human insulin and insulin analogues. Of note, molecular modifications of insulin analogues lead to shorter time to peak and duration of action, allowing close mimics of physiologic postprandial insulin action5.
Among newer therapeutics for T2D, GLP-1 receptor agonists (GLP-1RA) are widely used due to their attributes such as body weight loss, protection of islet beta-cells, promotion of islet beta-cell proliferation and minimal side effects. GLP-1RA mimic the action of endogenous GLP-1 to enhance insulin secretion and inhibit glucagon secretion from pancreatic islet cells6. Clinically, short-acting GLP-1RA are characterised by short-lived peaks in plasma drug concentrations following each injection, with intermittent periods of near-zero concentrations, whereas long-acting GLP-1 RA constantly elevates drug concentrations in a range leading to substantial GLP-1 receptor stimulation. While both classes of GLP-1RA can effectively reduce HbA1c, long-acting GLP-1RA are demonstrated to more profoundly lower fasting plasma glucose than short-acting GLP-1RA (Figure 1)7.
In addition to glycaemic control, GLP-1RA were demonstrated to reduce the risk of cardiovascular (CV) events, improving microvascular function, and reducing inflammation8. Moreover, GLP-1RA has been reported to lower body weight by their influence on the central nervous system7.
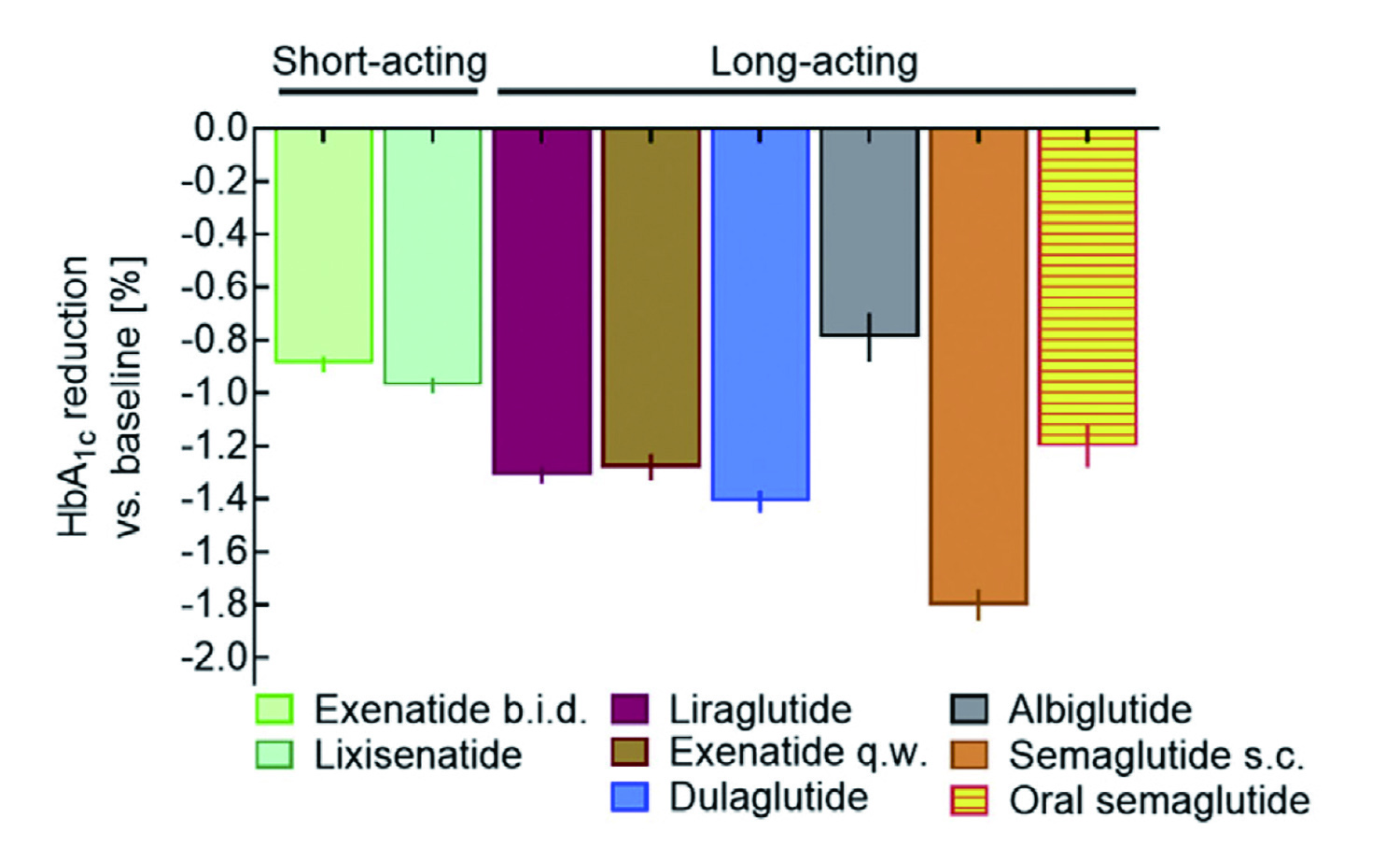
Figure 1. Comparative effectiveness of GLP-1RA in reducing HbA1c7
Apart from GLP-1RA, sodium-glucose cotransporters 2 inhibitors (SGLT2i) are also effective in glycaemic control with CV protective benefit documented. SGLT2i are a class of therapy for T2D which lowers glucose burden by inhibiting the major transporter involved in the glucose reabsorption in kidney and hence increasing urinary glucose excretion9. The mechanism of action for SGLT2i is insulin-independent that there is less risk of major hypoglycaemic events as compared to agents dependent on insulin action10. Notably, increased excretion of glucose and associated calories by the action of SGLT2i would decrease body weight11. Similar to GLP-1RA, SGLT2i have been reported to provide CV benefits. A recent meta-analysis by Kaze et al (2022) showed that SGLT2i were associated with reduced risks of major adverse cardiovascular events (MACE), kidney outcomes, hospitalisations for heart failure, and death among adults with diabetic kidney disease12.
Optimising T2D Outcomes by Personalised Management
Besides glycaemic presentations, T2D is associated with various non-glycaemic diseases leading to morbidity and mortality, including obesity, dyslipidaemia, hypertension, non-alcoholic fatty liver disease (NAFLD), and etc. Nonetheless, conventional T2D paradigm is primarily gluco-centric that views hyperglycaemia as T2D primary pathology, and glycaemic control its ultimate treatment13. In spite of the wide spectrum of treatment benefits of various classes of pharmacotherapies for T2D, former trials reported that about 40% of T2D patients failed to reach glycaemic control (HbA1c <7%)14,15.
In recent years, personalised medicine has been advocated as the most promising strategy in treating complicated polygenic diseases like T2D, because of variability in phenotypes across population groups and the need to determine the appropriate medication for each individual16. Personalised diabetes management is based on developing a clinical plan that is tailored to the individual. The approach takes into account many complex factors including the patient’s attitude, age, diabetes duration, diabetes medical history and comorbidities, and estimated life expectancy, as well as healthcare resources and support systems17.
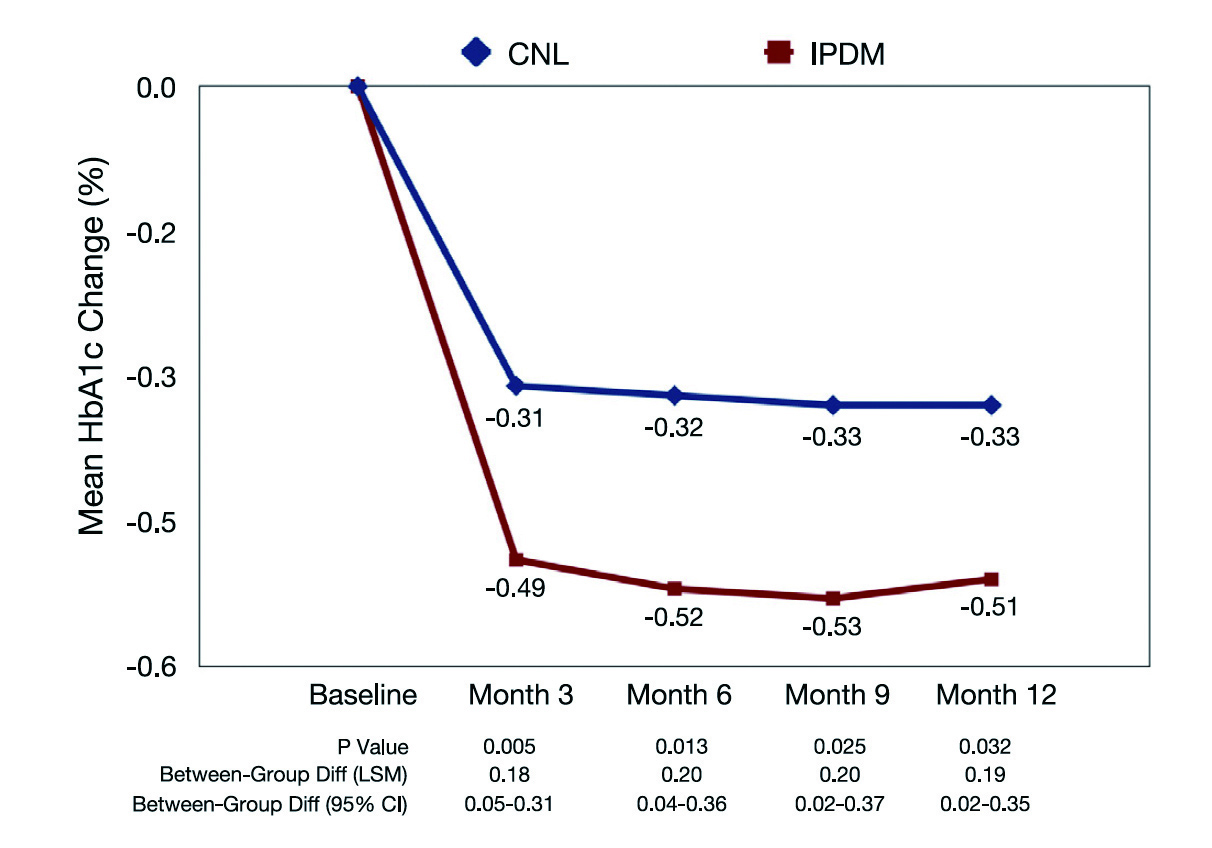
Figure 2. Change in HbA1c from baseline18, CNL: control
The clinical outcomes of integrated personalised diabetes management (IPDM) were evaluated in a clinical trial by Kulzer et al (2018) involving 907 patients with T2D. The patients were randomly assigned to receive usual care or IPDM process, which included structured assessment, structured and therapy-adapted self-measured blood glucose, and personalised treatment based on systematic analysis of patient’s data. Importantly, the treatment protocol was defined according to the discussion between physician and the patient. The outcome measures were measured after 12 months of follow-up18.
The results showed that IPDM led to a significantly greater reduction in HbA1c as compared to usual care (Figure 2). The results also indicated that the percentage of patients with therapy adjustments was higher in the IPDM group at all visits. Essentially, better patient adherence at month 12 was achieved with IPDM (odds ratio: 2.39, p = 0.0003). Moreover, higher satisfaction of both patients and physicians was generated with IPDM18. Thus, the findings suggested that personalised management in T2D improved the use of diagnostic data leading to better glycaemic control, more timely treatment adjustments, as well as improved patient adherence and enhanced treatment satisfaction.
Prospects in Personalised Medicine in T2D
In the personalisation of T2D management, a wide scope of medical and personal factors has to be taken into account. Key medical factors include the diabetes phenotype, available biomarkers and the presence of medical comorbidities. Moreover, patient factors such as treatment preference, age and life expectancy, diabetes duration, psychosocial concerns should also be considered when planning a holistic approach to patient management17. In particular, the roles of genetic biomarkers in predicting the risk of developing diabetic complications have been highlighted in previous investigations19. Hence, the use of big data generated by genome-wide association studies and the “omics”, including proteomics, metabolomics, and transcriptomics, along with advanced high-throughput sequencing technologies, will provide a promising future in precision medicine for diabetes. While the heterogeneous nature of diabetes poses great challenges for tailored disease management and addressing unmet patient needs, artificial intelligence (AI) and machine learning (ML) techniques would potentially facilitate the implementation of personalised medicine of T2D into clinical practice.
References
1.V55.: 1441-14 2021; Pharmgenomics Pers Med Venkatachalapathy et al. 19. : 200-V12.144 2018; Diabetes Res Clin Pract Kulzer et al. 18. : 281-V95.15 2022; Metab Syndr Obes Targets Ther Williams et al. Diabetes, 17. : 354.7 2016; World J Diabetes Marín-Peñalver et al. 16. : 1376-V85.179 2019; JAMA Intern Med Kazemian et al. 15. : 47-V56.80 2014; Clin Endocrinol (Oxf) dePablos-Velasco et al. 14. : 613-V29.21 2020; Rev Endocr Metab Disord Bar-Tana. 13. : 1-V14.21 2022; Cardiovasc Diabetol Kaze et al. 12. : 1020-V31.97 2012; J Clin Endocrinol Metab Bolinder et al. 11. : 136-V42.27 2010; Diabet Med Gerich. 10. : S20-V7.79 2011; Kidney Int List et al. 9. : 1040.12 2021; Front Endocrinol (Lausanne) Zhao et al. 8. : 101102.46 2021; Mol Metab Nauck et al. 7. : 1767.12 2021; Front Pharmacol Yang et al. 6. : 13-V42.78 2018; Metabolism Upadhyay et al. 5. : 405-V12.321 2000; BMJ Stratton et al. 4. : 1249-V58.44 1995; Diabetes U.K. prospective diabetes study 16.. 3. V67.: 551-13 2022; Diabetes Ther DiLoreto et al. 2. . DOI:10.1016/J.DIABRES.2021.109119.183 2022; Diabetes Res Clin Pract Sun et al.





