
The Need for a More Holistic BC Screening Practice
BC is the most common cancer among women and the third leading cause of female cancer mortality in Hong Kong8. Approximately 50% of breast tumours can double in up to 25 days, reflecting a rapidly progressive course of BC associated with worse prognosis1. In fact, according to a retrospective study of the Hong Kong Cancer Registry, while the relative 5-year survival rate for stage I and stage II diseases could reach 97.5% and 87.6% respectively, it plummeted to 66.2% for stage III and a mere 19.3% for stage IV2. Although the survival rates of early BC are relatively higher, it is unfortunately difficult to detect the early-stage disease due to its asymptomatic nature2,3. Therefore, to achieve better treatment outcomes while extending patients’ survival, early BC screening for the asymptomatic disease remains imperative for the female population, especially for women with risk factors of BC, such as early menarche (<12 years old) or late menopause (>55 years old)4,9.
The Centre for Health Protection (CHP) has recommended different methods for early detection or screening4. Individually, women are advised to be breast aware by familiarizing themselves with the normal look and feel of their own breasts, and visiting doctors promptly in case of suspicious symptoms noted4. While breast awareness appears to be readily performed, it could be highly subjective and of questionable reliability as BC knowledge varies from woman to woman. In fact, a Hong Kong survey has revealed that the majority (62.6%) of women were reportedly not confident in noticing breast changes10.
On the other hand, 2-dimensional MMG remains a clinical standard for BC screening4. CHP recommends that women be screened annually or biennially, depending on their BC risk level4. However, the considerable time gap between screenings may leave women uncovered during this period. Irvin et al. have reported that interval BCs diagnosed within 1 year after a negative MMG result but prior to the next scheduled screening were significantly associated with an unadjusted BC-related mortality risk of 1.92 times compared with BCs detected at screening (HR=1.92; 95% CI: 1.39-2.65; p<0.001) (Figure 1)11. Thus, additional interventions during this period may be necessary to minimize the mortality risk11.
Collectively, it highlights that there is room to further optimize the current screening practice with the incorporation of newer and complementary screening modalities for providing a scientific, easily accessible and continuous monitoring of women’s breast health.

Figure 1. BC-specific mortality stratified by interval BC vs BC detected by screening11.
Elevated Breast Temperature as a Possible Early Sign of BC Development
In recent decades, the use of thermography to detect early breast abnormalities has gained increasing grounds, thanks to the numerous clinical studies confirming the hyperthermic skin feature of BC12. A couple of theories have been suggested to drive the temperature increase of BC tissues12. Firstly, cancer cells prefer aerobic glycolysis over mitochondrial oxidative phosphorylation for energy production, a process known as the Warburg effect12. Due to the inefficiency of aerobic glycolysis, the metabolic rate of cancer cells is compensatively increased, thus resulting in increased metabolic heat generation12. Secondly, tumour growth is supported by angiogenesis, which increases blood flow to tumour cells and hence elevates the local temperature13. Consequently, BC cells may exhibit their own independent thermal signatures that deviate from the circadian rhythm of normal breast tissues, making their thermal pattern a distinct feature predictive of BC risk (Figure 2)7,13.
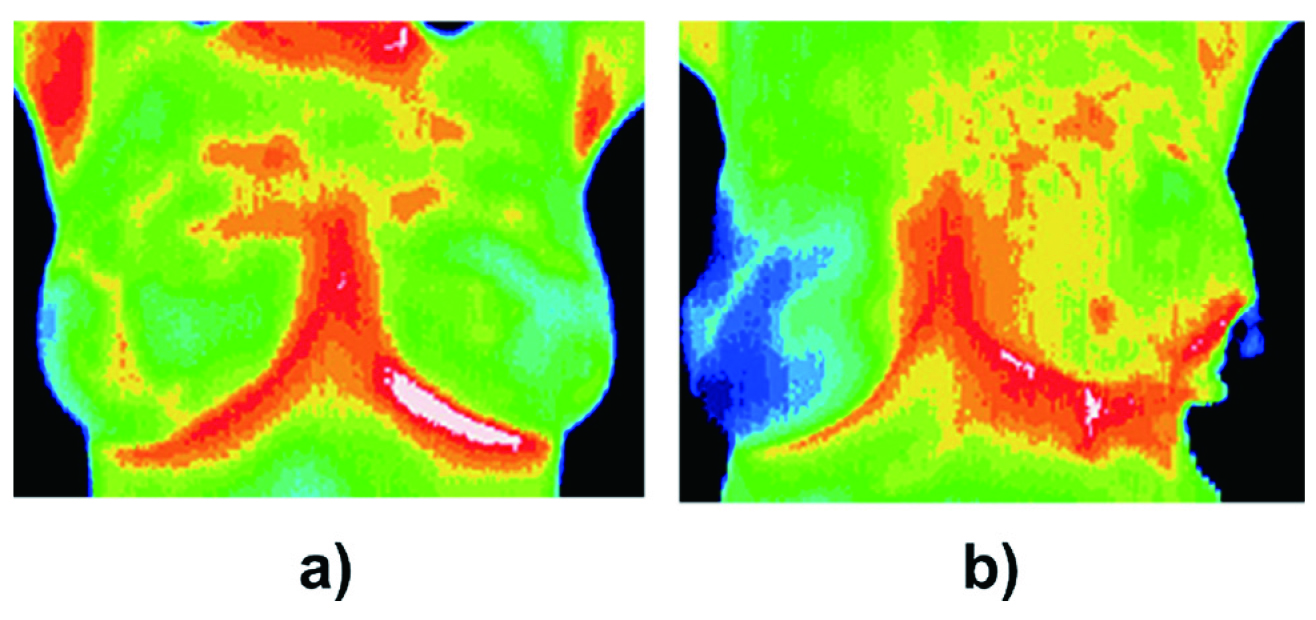
Figure 2. Thermograms of (a) normal breast, (b) with BC in the upper outer quadrant of the left breast5.
Leveraging these science principles, different thermographic techniques have been developed throughout the years5. While the conventional steady state thermography may be subjected to high false-positive and false-negative rates with low sensitivities, the succeeding dynamic thermal analysis has shown improvements in sensitivities and false-positive rates, demonstrating its clinical value and applicability5. With the advancement of technology, more convenient and delicate thermographic detection device in the form of wearable bra that is non-invasive, non-radiating and non-compressive may have become commercially available as well6,7.
Convenient and Portable Wearable Devices for Breast Health Monitoring
These devices may be equipped with multiple thermal sensors that continuously collect skin surface thermodynamic metabolic data at various breast locations for an extended period of time, such as during sleep6,7. Once the data are recorded, an affiliated mobile application can help download the data from the sensors and transfer them to a cloud database for analysis using automated predictive artificial intelligence algorithms or specially developed statistical software6,7. Once analysed, graphs reflecting longitudinal changes such as temperature points of each contra-lateral breast are plotted against each other to create a thermal pattern for viewing potential differences suggestive of breast lesions (Figure 3)6,7. Clinicians can then continuously monitor users’ breast changes by studying these graphs via the mobile application6,7.
Clinically, these portable breast health monitoring devices have been well tested. For instance, in a wearable device designed to detect breast thermal pattern deformity and tissue elasticity, after feeding the data of 150 women for the analytic algorithms, the sensitivity of identifying BC could reach 89%14. In another study enrolling 93 females with benign breast tumours and 108 with malignant breast tumours, the subjects were required to wear two biometric patches to collect 24-hour breast skin temperature circadian rhythm data for analysis7. The predictive model with these collected data was able to differentiate benign and malignant lesions, demonstrating 78% accuracy, 83.6% sensitivity and 71.5% specificity7. The investigators concluded that the wearable device may prove valuable for breast health monitoring as well as identifying possible abnormalities under clinicians’ supervision7.
Conclusion
BC screening serves as an important element in identifying potential BC development early. Despite breast awareness and MMG being recommended approaches or clinical gold standards, the existing detection and screening practice may not be fully optimized yet, thus requiring additional interventions. The emerging wearable devices that provide continuous breast health monitoring based on heat signatures could be a convenient and sensitive tool for both patients and clinicians in exposing potential early breast malignancy and facilitating the overall management of BC.
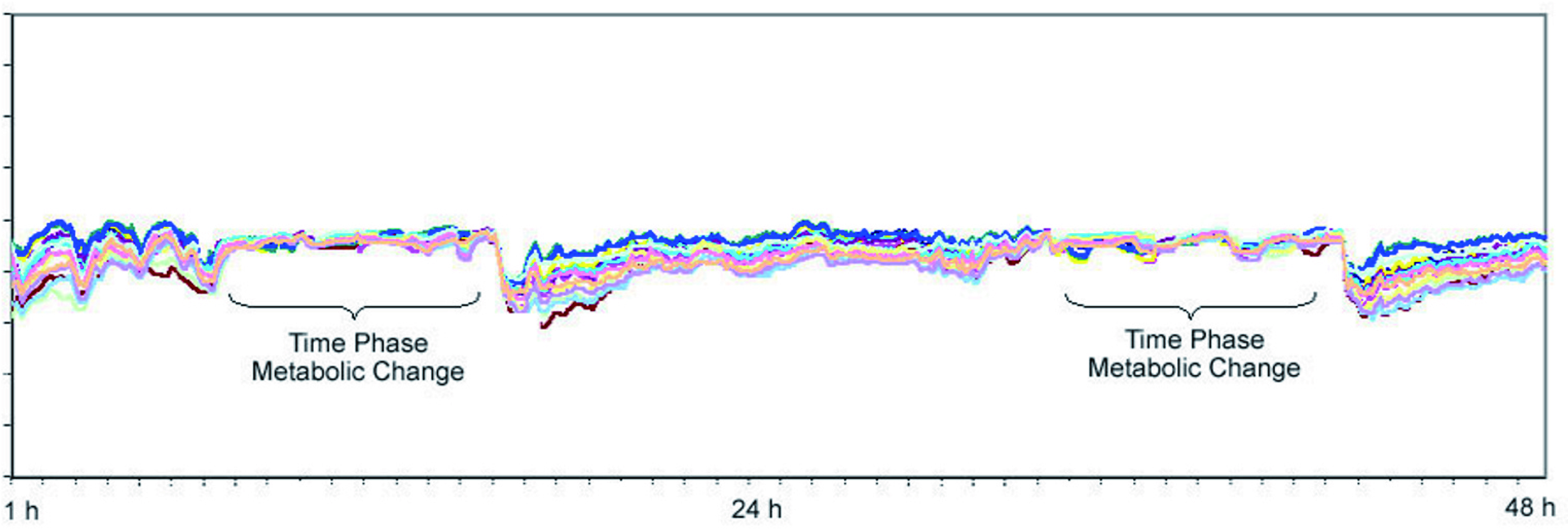
Figure 3. Dynamic thermal analysis in a patient with T1 BC6.
References
1. Pearlman AW. Cancer. 1976;38(4):1826-1833. 2. Kwong A, et al. Ann Surg Oncol. 2011;18(11):3072-3078. 3. Khan S, et al. Multimed Tools Appl. 2017;76(1):33-57. 4. Centre for Health Protection. Cancer Expert Working Group on Cancer Prevention and Screening (CEWG). Recommendations on Prevention and Screening for Breast Cancer For Health Professionals. Available at: https://www.chp.gov.hk/files/pdf/breast_cancer_professional_hp.pdf. Accessed Aug 11, 2022. 5. Gonzalez-Hernandez JL, et al. Int J Heat Mass Transf. 2019;131:558-573. 6. Salhab M, et al. Int Semin Surg Oncol. 2006;3:8. 7. Royea R, et al. Comput Methods Programs Biomed. 2020;197:105758. 8. Centre for Health Protection. Breast Cancer. Available at: https://www.chp.gov.hk/en/healthtopics/content/25/53.html. Accessed Aug 4, 2022. 9. Hong Kong Breast Cancer Registry Bulletin Issue 9: Risk factors for Breast Cancer in HK Women: a Case-Control Study, published by Hong Kong Breast Cancer Foundation in September 2018. 10. Yeung MP, et al. World J Clin Oncol. 2019;10(2):98-109. 11. Irvin VL, et al. JAMA Netw Open. 2020;3(6):e207227. 12. Lozano III A, et al. Bioengineering. 2021;8(7):86. 13. Salhab M, et al. Int Semin Surg Oncol. 2005;2(1):8. 14. Bhavya G, et al. A study on personalized early detection of breast cancer using modern technology. In Emerging Research in Electronics, Computer Science and Technology 2019 (pp. 355-362). Springer, Singapore.





