
The Therapeutic Potential of Alpha-2-Macroglobulin
in Controlling Osteoarthritis
Osteoarthritis (OA) is an aging-related chronic degenerative disease characterised by the degradation of chondrocyte extracellular matrix. The disease is one of the most common causes of disability in elderly. Essentially, it has been reported that the prevalence of radiographic knee OA among Chinese women was 42.8% and 21.5% among men, whereas symptomatic OA occurred in 15% of women and 5.6% of men1. With the impact of population aging, the health burden of OA is expected to be intensified. In addition to conservative treatments including lifestyle modification and physiotherapy, total joint replacement is effective in relieving symptoms of knee OA2. Nonetheless, non-surgical alternative therapy for OA is highly desirable. Notably, alpha-2-macroglobulin (A2M) has been demonstrated to have excellent anti-inflammation effect with preferable safety profile and hence would be a promising strategy countering OA.
A2M - The Master Inhibitor of Cartilage Degrading Factors
OA progression is, at least in part, due to the up-regulation of inflammatory mediators and proteases3. As elevated levels of catabolic enzymes in synovial fluid (SF) are associated with chrondrocyte death and cartilage matrix degeneration, molecular interventions targeting these enzymes would potentially arrest the progression of OA and preserve joint health.
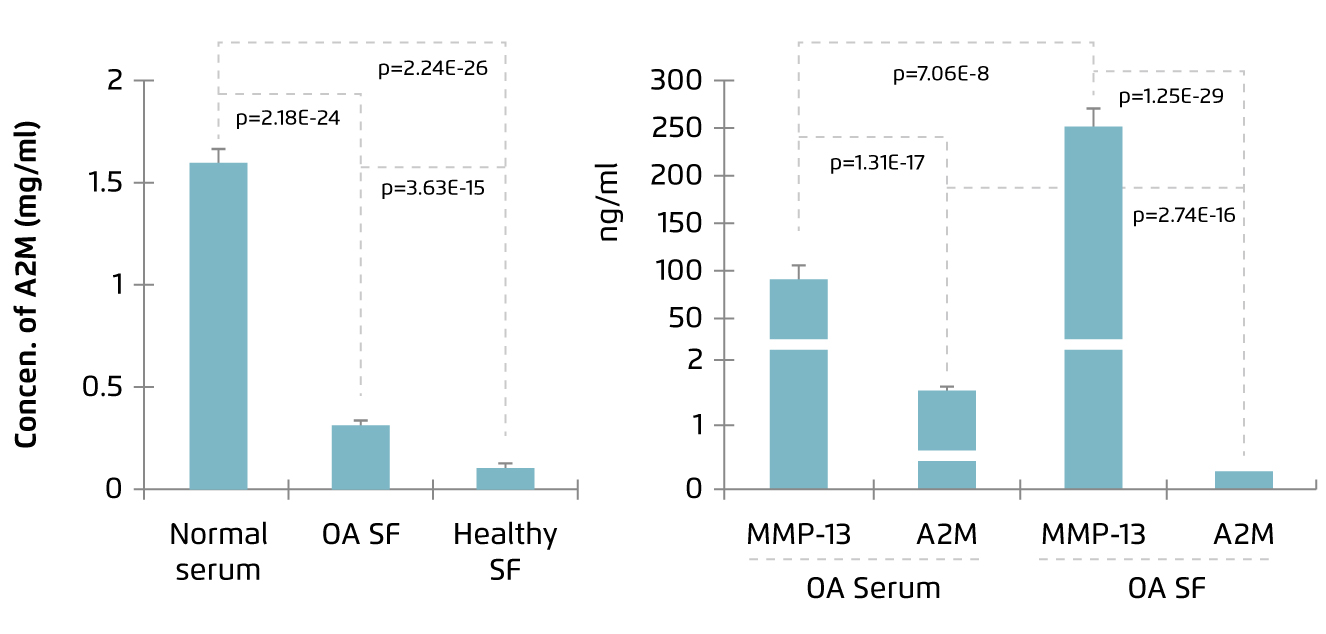
Human A2M is a 720 kDa homotetrameric protein abundant in plasma. It is a protease inhibitor regulating various proteolytic processes and is involved in innate immunity. A2M has been demonstrated to inhibit proteases involving in coagulation and fibrinolysis, inflammatory proteases, and proteases which remodel extracellular matrix, such as matrix metalloproteinases (MMPs), in vitro. Besides, A2M can inhibit pathogen-expressed proteases as well4. Thus, in view of its inhibitory action on cartilage catabolic enzymes, A2M has been identified as a potential therapeutic agent for controlling OA. However, Wang et al (2014) reported that the level of A2M was lower in SF as compared to serum in both normal and OA subjects (Figure 1)5, whereas increased level of MMP-13, the primary MMP involved in cartilage degradation6, in SF compared with serum was observed in OA patients (Figure 2)5. This fact has led to the investigations on therapeutic efficacy of intraarticular injection of A2M in controlling OA progression.
|
Figure 1. A2M level in SF and serum5 |
Figure 2. Increased MMP-13 level in OA SF5 |
Attenuation of Cartilage Damage in Arthritis
The effectiveness of A2M in attenuating cartilage degeneration has been evaluated in former preclinical studies. For instance, Zhang et al (2017) demonstrated that intraarticular injection of A2M at 24 hours and 2 weeks after anterior cruciate ligament transection (ACLT) significantly reduced inflammation and cartilage degeneration, as represented by histological scoring and Osteoarthritis Research Society International (OARSI) score respectively, associated with ACLT-induced OA after 8 weeks in rat model. Of importance, lower levels of MMP-13 and collagen-II degraded product, and stronger collagen-II synthesis were detected in animals treated with A2M as compared to phosphate-buffered saline (PBS)-treated controls7.
More recently, Li et al (2019) showed that intraarticular injection of A2M of different concentrations would exert an anti-inflammatory effect and attenuate cartilage and bone damage in collagen-II induced arthritis (CIA) in mice model. Briefly, clinical symptoms of arthritis including redness and swelling were developed in all animals after induction treatment. 3 doses of intraarticular A2M injections were performed, with 1-week interval, after developing OA symptoms, whereas animals were sacrificed 1 week after the last injection. Histological results indicated that A2M treatment yielded lower levels of inflammatory infiltration and synovial hyperplasia, as reflected by inflammation scores, compared to PBS-treated control (Figure 3). Moreover, the results also indicated that inflammation-related gene expression such as MMP-3, -9, -13 and Runx2 were significantly less expressed in A2M-treated groups than PBS-treated animals8. Hence, preclinical data suggested that intraarticular injection of A2M is effective in countering inflammation and attenuating cartilage degeneration in induced OA models.
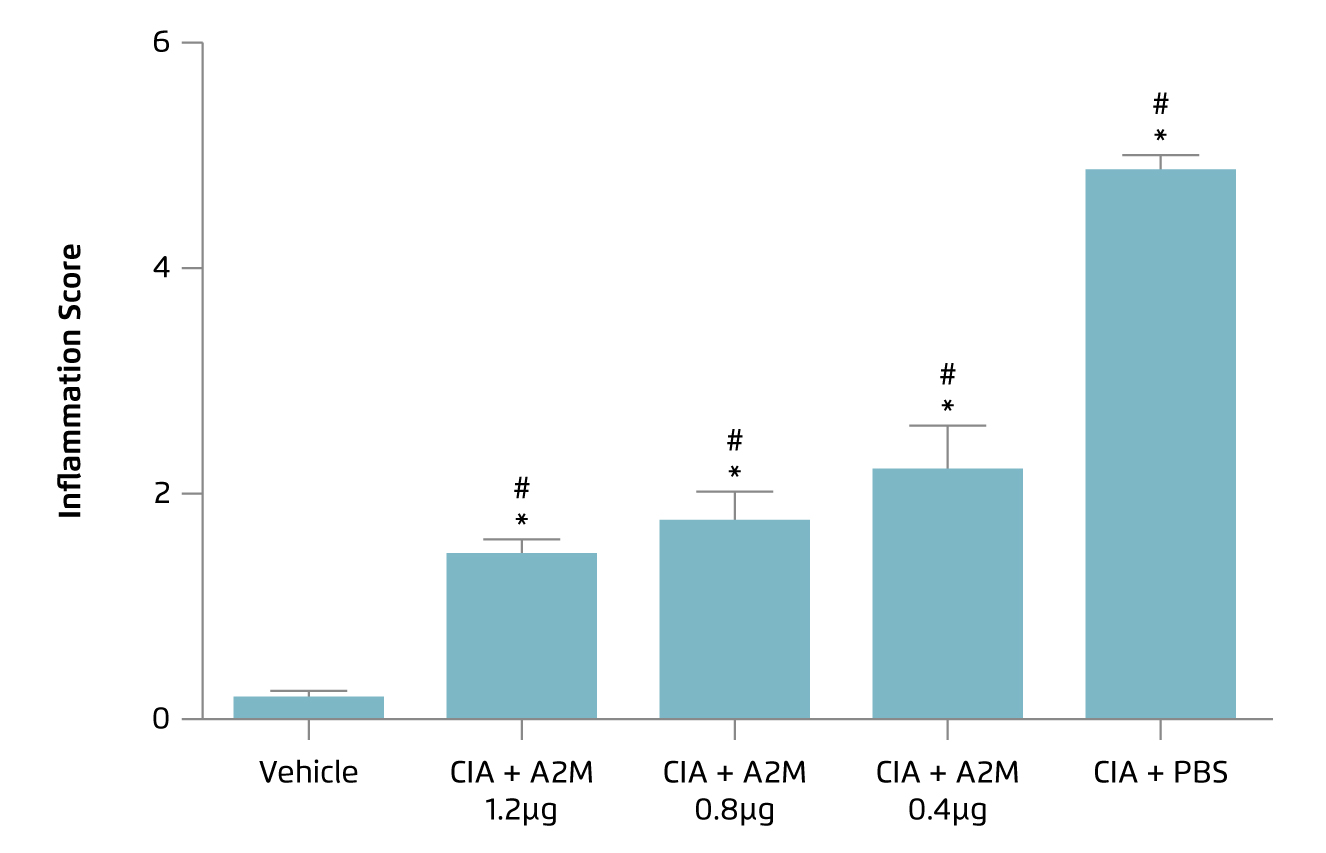
Figure 3.
Inflammation score achieved by different concentrations of A2M8, *p < 0.05 versus vehicle group; #p <0.05 versus CIA+PBS group
Clinical Benefits of A2M in OA
Apart from preclinical studies, clinical trials investigating the efficacy of A2M in human have been conducted. For instance, in a prospective randomised control trial (RCT) by Thompson et al (2021), 75 patients with knee OA were randomly allocated to receive intraarticular injection of A2M, platelet-rich plasma (PRP) or corticosteroids. Patient reported outcomes were collected prior to injection, 6 weeks and 12 weeks following injection. In addition, clinical assessment including the visual analog scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Knee Injury and Osteoarthritis Outcome Score (KOOS), Lysholm, and Tegner scores were measured as well.
The results demonstrated that A2M treatment yielded the greatest trend of improvement in VAS and WOMAC scores at week 6. Further, at week 12, significant improvement in WOMAC score was observed in A2M group as compared to traditional PRP treatment (-18.43 vs. -5.70, p <0.02). Although the difference was not statistically significant, corticosteroid (-1.57) and A2M (-1.70) generated better improvements in VAS compared with PRP (-0.61)9. The results thus suggested that A2M treatment exhibits comparable effectiveness to corticosteroid in controlling knee OA, whereas the improvements yielded by A2M and corticosteroid were better than PRP.
The Non-surgical Alternative for OA
Patients with OA are usually in excruciating pain and have significant functional impairment. Former opinions commented that long delay before surgical treatment would lead to deterioration in terms of pain, functionality, and health-related quality of life, which could eventually affect post-surgery outcomes2. This highlighted the clinical importance of developing alternative therapies to control OA. Established evidence suggesting that A2M treatment would downregulate damaging factors in cartilage catabolic process and hence is protective for cartilage against OA progression. Nonetheless, further optimization in the A2M preparation process would facilitate its routine application in the management of OA.
References
1. Zhang et al. Arthritis Rheumatol 2001; 44: 2065-71. 2. Yuen. Hong Kong Med J 2014; 20: 5-6. 3. Kim et al. Rheumatol Int 2010 314 2010; 31: 543-7. 4. Harwood et al. J Biol Chem 2021; 297: 100879. 5. Wang et al. Arthritis Rheumatol 2014; 66: 1843-53. 6. Hu et al. Int J Mol Sci 2021; 22: 1-22. 7. Zhang et al. Arthritis Res Ther 2017; 19. DOI:10.1186/S13075-017-1363-4. 8. Li et al. Int J Rheum Dis 2019; 22: 654. 9. Thompson et al. Arthroscopy 2021; 37: e80





