Novel Antiviral Mechanism Targeting Pressurised DNA State in Herpesvirus
Herpes simplex virus (HSV) is a highly contagious viral infection prevalent across the world. With HSV-1 and HSV-2 being the most common types, approximately 90% of people worldwide have had infections of one or both viruses1. HSV is transmitted upon close contact and mostly infects the skin and mucosal surfaces, especially at the oral and genital areas2. Patient outcomes can be highly variable depending on host immune reactivity. While immunocompetent hosts may show relatively mild symptoms like lesions or blisters at infected areas, immunocompromised hosts are often associated with more severe clinical manifestations and much higher risk of recurrent infections1. Notably, current anti-herpes treatments are effective, but anti-herpes drugs have frequently selected for drug-resistant strains after long-term use3. Recently, a new antiviral target of suppressing pressure-driven viral genome ejection by HSV is likely impervious to developing drug resistance4. This target hence represents a platform for discovery of a new class of therapies for HSV and other viral infections with genome-pressure-dependent replication.
Current Challenges in HSV Treatment
Acyclovir (ACV) and penciclovir (PCV) are two common antivirals used by HSV patients. Both are activated upon intracellular phosphorylation by viral thymidine kinase (TK), and subsequently bind viral DNA chains to inhibit replication (Figure 1)5. However, the gene that codes for viral TK is subject to mutations which can give rise to decreased levels or altered specificity of the enzyme6. Patients may then develop drug-resistant HSV and are no longer responsive to antivirals that utilise TK. On the other hand, foscarnet was developed as a form of salvage therapy which directly inhibits viral DNA polymerase without relying on intracellular phosphorylation and viral TK. Yet, mutations in the viral DNA polymerase gene can also render the drug ineffective7.
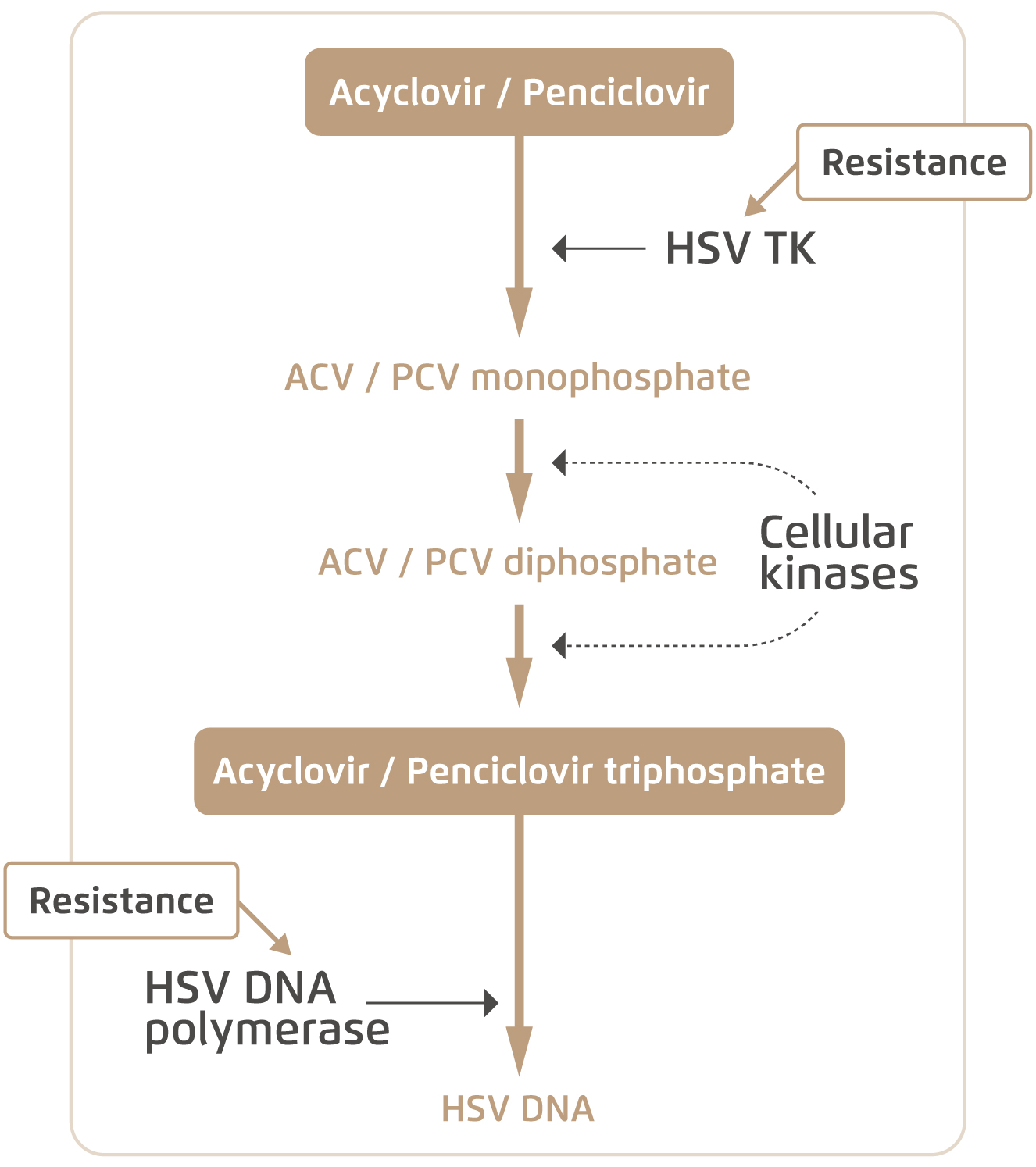
Figure 1. Intracellular mechanism of common anti-HSV drugs and resistance5
It is apparent that resistance presents a major clinical hurdle in antiviral development. Notably, immunocompromised patients are particularly vulnerable to developing resistance due to higher viral loads and greater instances of mutation. They are more susceptible to more severe HSV infections and complications like meningoencephalitis and pneumonia that can be life-threatening. Thus, HSV patients often require continuous monitoring of drug efficacy and detection of resistance, which is a labour-intensive and time-consuming process in the clinical setting. A novel therapy overcoming drug resistance will undoubtedly be favourable in combatting HSV infections and hence improving long-term health outcomes for patients infected with HSV.
Resistance-Free Mechanism against HSV Infection
The general problem with traditional antivirals lies in their over-reliance on targeting viral proteins and viral replication. Any mutations and structural changes to these proteins will usually result in resistance. Recently, a novel antiviral mechanism of action (MOA) against HSV was discovered. The mechanism exploits the physical nature of viral particles with regards to DNA-DNA electrostatic and steric interactions within their genomes4. Since these forces are intrinsic properties of the virus, they are not prone to alterations or mutations and thus unlikely to develop resistance.
Previous studies demonstrated that herpesvirus has a tightly-packed genome within its capsid, and repulsive forces between negatively-charged DNA generate immense pressure against the capsid wall. This pressurised state is what powers viral ejection of DNA into an infected host cell nucleus8. Hence, the primary goal of this novel MOA is to neutralise repulsion between viral DNA strands and remove intracapsid pressure. Three polycationic compounds (Arg5+, bPEI 600, DAB-Am-4) have been identified to effectively condense DNA upon binding, thereby replacing DNA-DNA repulsion with attractive forces and in turn lowering pressure against capsid wall4. This can effectively suppress viral ejection and protect the host cell from being infected.
Breakthrough Research Demonstrates Antiviral Efficacy
The first evaluation on the antiviral potential of the 3 candidate compounds is their ability to penetrate viral capsid walls and condense the DNA inside. Remarkably, molecular weight is an important factor governing the efficacy of these compounds. It has been tested that larger compounds, even with greater condensing power, failed to achieve viral DNA condensation due to their inability to enter the intracapsid space4. In the trial, all three selected compounds were able to permeate whole virions and condense viral DNA to the desirable levels of under 31Å at physiologically relevant temperatures. This is evident based on measuring intracapsid DNA-DNA spacing (Figure 2)4. The next evaluation focused on the extent of antiviral activity of the compounds with the specific condensing power. The results indicated that the 3 compounds can effectively lower intracapsid pressure and suppress viral genome ejection into host cell nucleus.
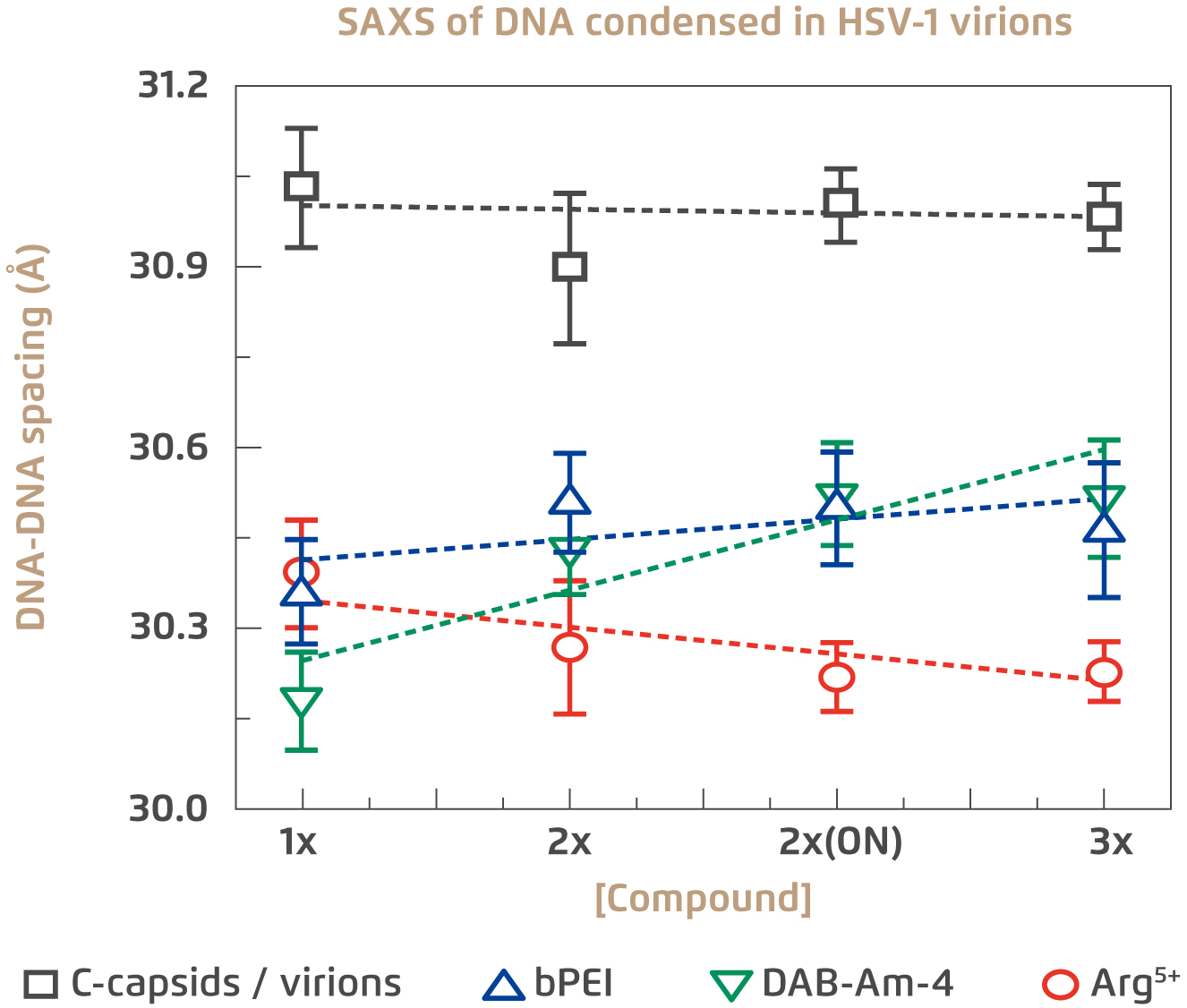
Figure 2.
Extent of DNA condensation in HSV-1 virions by the three selected compounds4
SAXS: Small-angle X-ray scattering, ON: overnight
Figure 3 shows the electronic microscopic images of condensed DNA in viral capsid upon treatment with the 3 selected compounds, whereas viral DNA was ejected leaving the empty capsid in the untreated control4.
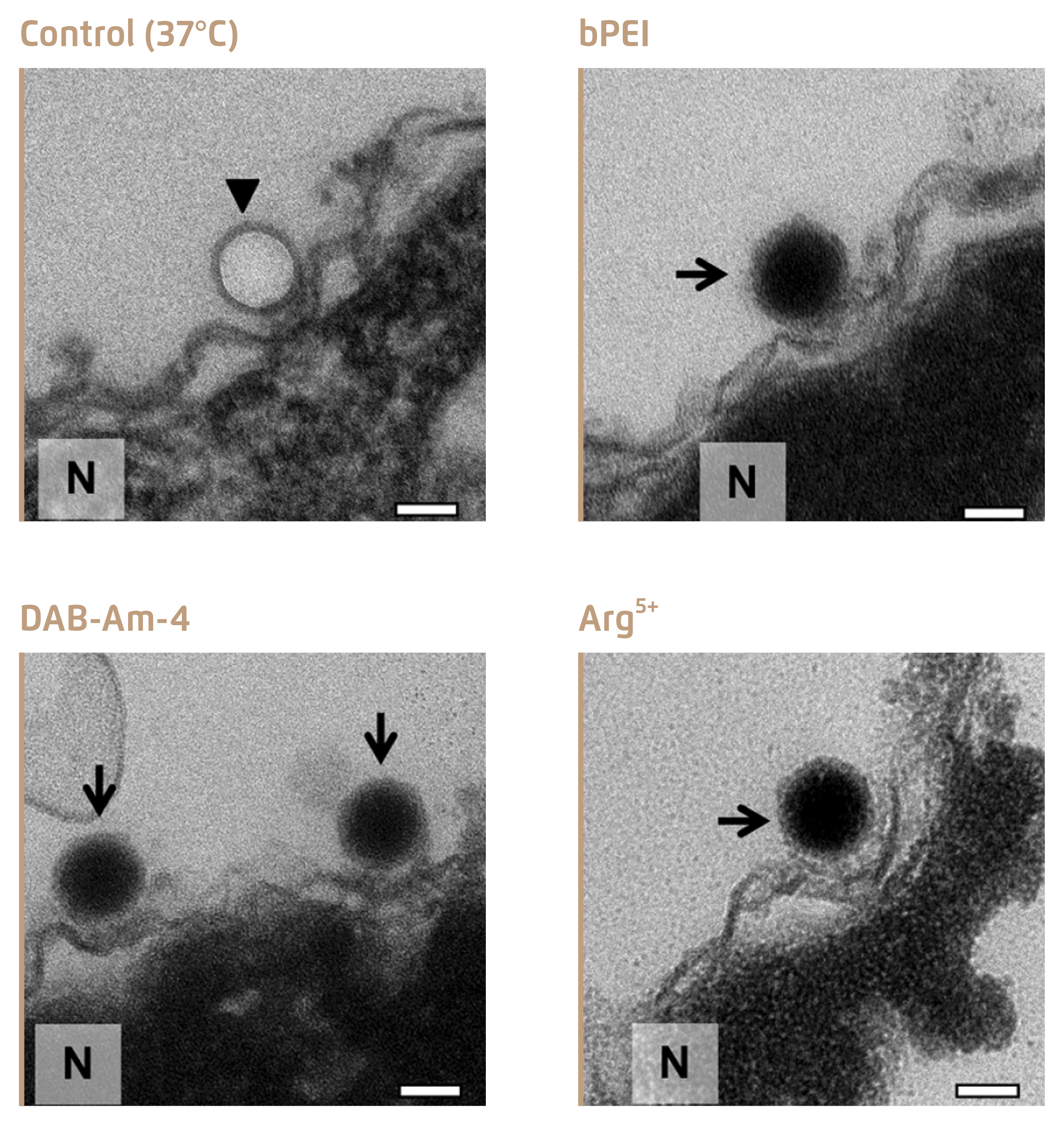
Figure 3.
Inhibition of DNA ejection from HSV-1 C-capsids4, bold arrows show empty capsids that ejected DNA, and thin arrows show DNA-filled capsids with DNA condensed inside
Clinical Implications and Future Perspectives
The MOA of targeting the pressurised DNA state serves as a new strategy in antiviral development, which would potentially tackle the clinical obstacle of drug resistance of HSV. In particular, the three identified compounds, Arg5+, bPEI 600 and DAB-Am-4, were demonstrated to effectively inhibit ejection of viral DNA into host cells. Preclinical tests on mice further showed that these compounds were well-tolerated with low cytotoxicity4. More importantly, this novel MOA is expected to be applicable as a broad-spectrum treatment against all HSV infections. Future investigations on implementing this MOA on other common virus types such as HIV and Hepatitis B virus would facilitate the development of therapeutics combatting these infections.
Acknowledgement
The author appreciates the editorial support by Mr Gavin Chiu of the School of Biomedical Sciences, CUHK in the preparation of this article.
References
1. Wald et al. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press, 2007: 656-72. 2. Whitley et al. Pathogenesis and disease. Cambridge University Press, 2007 3. Jiang et al. Int. J. Oral Sci. 2016; 8: 1-6. 4. Brandariz-Nuñez et al. PLoS Pathog 2020; 16: e1008604. 5. Bacon et al. Clin. Microbiol. Rev. 2003; 16: 114-28. 6. Morfin et al. J. Clin. Virol. 2003; 26: 29-37. 7. Paintsil et alEncyclopedia of Microbiology. Elsevier Inc., 2009: 223-57.
8. Bauer et al. J Am Chem Soc 2013; 135: 11216-21.





