The Pathophysiological Significance of Neuronal Regulation in Development of Tendinosis
Tendinopathy is a clinical condition characterised by pain and swelling in and around tendons, whereas tendinosis is a chronic, degenerative condition with objective changes in tendon structure, often seen in patients with tendinopathy1. Achilles tendon, especially among athletes, is vulnerable to developing tendinosis upon continuous, prolonged and intense functional demands imposed on the tissue2. Although the regulation of tendon homeostasis is still not fully understood, emerging data suggesting an active role played by the nervous system, via specific neuronal mediators, in regulating pain, inflammation and tendon homeostasis. In particular, the involvement of substance P (Sub-P) in the development of tendinosis has gained attention. While Sub-P is associated with mediating pain, oedema and fibrosis in tendinopathy, strategies on regulating Sub-P would be of clinical interest.
Tendinosis - The Degenerative Process in Tendon #ee86a8
Tendinopathy refers to a complex multi-faceted pathology of the tendon characterised by pain, decline in function and reduced exercise tolerance3. Traditionally, tendinitis is the term used to describe painful symptoms attributed to inflammation of the tendon, whereas tendinosis is the degenerative process without histological or clinical signs of intra-tendinous inflammation4. Tendinosis is reported to be the outcome of higher rate of degeneration comparing with that of synthesis of cell matrix upon repetitive trauma induced by overuse, decreased blood supply and muscle imbalance or weakness2. Essentially, in the pathogenesis of tendinopathy, both inflammation and degeneration are likely to be involved and interact with each other.
As micro-damages to tendon fibres occur, a number of substances promoting tendon healing, which includes inflammatory factors, will be secreted in response to the harmful stimuli and to exert a protective effect on the tendon5. Nonetheless, chronic and unregulated inflammation is harmful. Previous opinion suggested that there is a continuous transition from physiological to pathological processes in the tendon. In the case of tendinosis, factors such as overuse can cause injury to tendon leading to inflammatory responses. Additionally, the damage caused by overuse also triggers non-inflammatory reactions, including tendon thickening and increasing hardness. If physical overuse persists, self-repair of tendon will decline leading to disordered tendon tissue6. Thus, one of the typical features of tendinosis is a reduction of collagen organisation accompanied by increased collagen turnover1. The histological changes in tendinosis, in terms of disorganised collagen fibres and hypercellularity, in rat Achilles tendons upon 8 weeks of forced running (1 hour/day) are displayed in Figure 1.
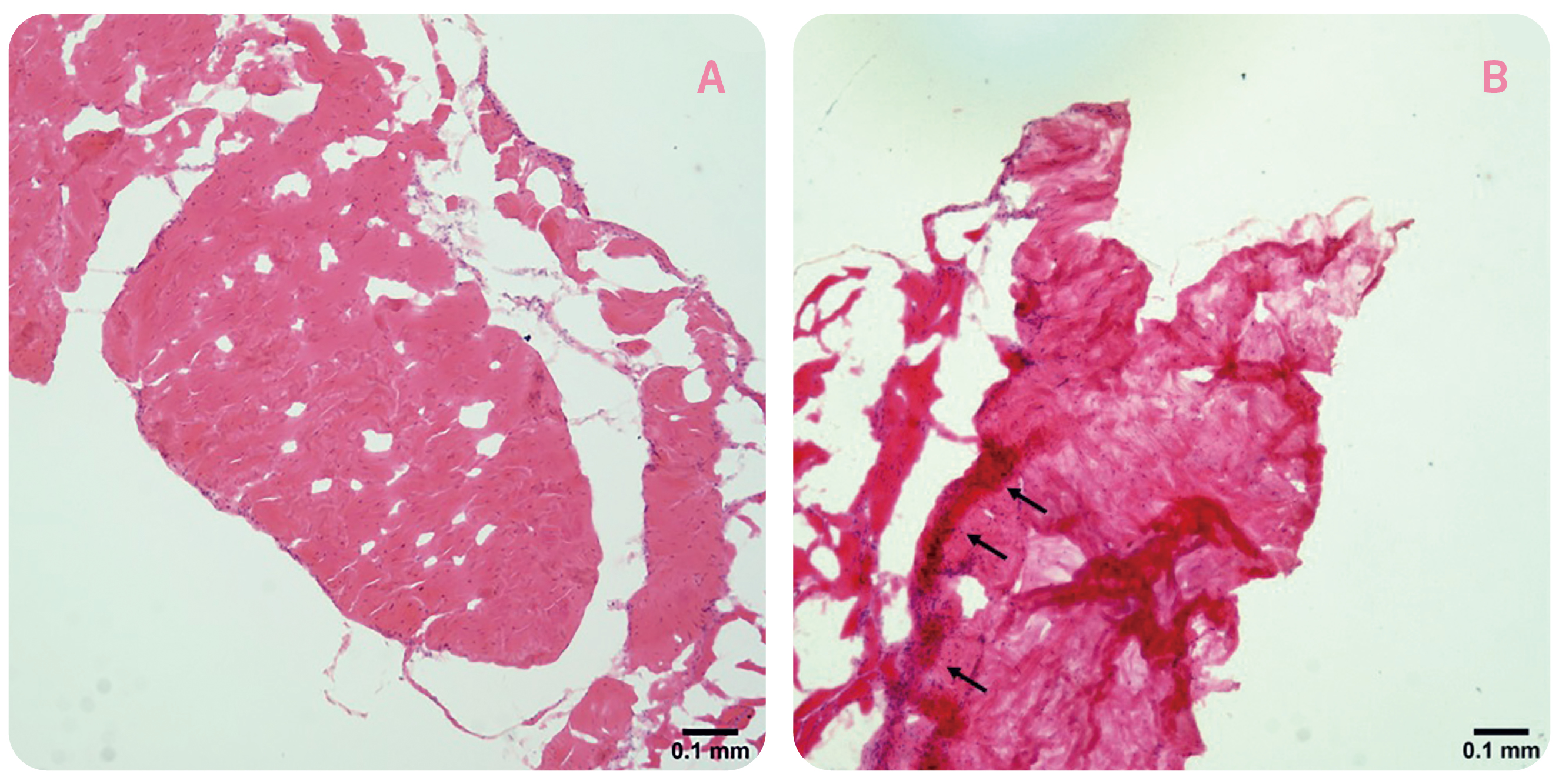
Figure 1. Histological changes in rat tendons after prolonged forced running, A) cage control, B) forced running group (arrows indicate the occurrence of hypercellularity)
Neuronal Regulation of Tendon Homeostasis
Tendons exhibit a low degree of innervation, which partly explain the slow adaptation to repetitive loading, prolonged healing and vulnerability to chronic injuries. Normally, nerve fibres do not enter the tendon, but terminate as nerve endings on different surfaces of the tendon. Mediated by different types of the nerve fibres innervated, mechanoception, nociception and vasomotor modulation are three of the main functions of tendon innervation7. In addition, there are established evidence on the participation of peripheral nervous system in the regulation of a wide variety of efferent actions including cell proliferation, inflammation and hormone release. In particular, neuronal regulation of tendon homoeostasis has received increasing attention.
The neuronal regulation of tendons has been shown to be mediated by three major groups of molecules including sensory, autonomic and excitatory neuromediators. In healthy tendons, the neuromediators are found in the surrounding structures. Nonetheless, former works have identified the occurrence of neuropeptides, including Sub-P and calcitonin gene-related peptides (CGRP), in tendons8. Upon binding to specific G-protein-coupled receptors, the neuropeptides generate various second messengers which trigger a wide range of effector mechanisms regulating cellular excitability and function. Notably, Sub-P is a potent vasodilator and have been demonstrated to exert pro-inflammatory effects, whereas anti-inflammatory neuromediators including galanin and somatostatin have been identified in vascular nerve fibres co-localised with Sub-P, suggesting modulatory and anti-inflammatory actions9,10. The balance between pro-and anti-inflammatory peptides is well-maintained in healthy tendons. However, upon tendon injury and during repair, there is extensive nerve ingrowth into the tendon proper, followed by a time-dependent emergence of sensory, autonomic and glutamatergic mediators, which amplify and fine-tune inflammation and regulate tendon regeneration. Moreover, in tendinopathic condition, excessive and protracted presence of sensory and glutamatergic neuromediators has been identified11.
Previous studies showed that denervation upon spinal cord injury would result in tendon alterations, which could partly be reversed by functional electrical stimulation12. On the other hand, diabetic patients are at higher risk of neuropathy while decreased levels of sensory neuropeptides are commonly observed13. Hence, existing findings support that the structure, function and healing capacity of tendons, as well as connective tissues such as ligaments, are influenced by nerve-derived factors.
The Involvement of Substance P in Tendinosis Development
Sub-P is a neuropeptide primarily known for its pro-inflammatory effects and effects on vasodilation and vascular permeabilisation14. It is also a pain-producing substance, probably associated with pain in tendon diseases6. There are findings suggesting the role of Sub-P in the progression of tendinopathy. For instance, increased expression of Sub-P-immunoreactive nerve fibres and increased mast cell number, which could be influenced by Sub-P, are observed in tendinosis15. Andersson et al (2011) demonstrated in an overuse animal model that injection of Sub-P accelerated the development of tendinosis-like changes. The results also found that paratendinous inflammation was more pronounced in the Sub-P-injected group than in the saline-injected controls14.
While being an important component in the development of tendinopathy, Sub-P is reported to play a critical role in tendon healing that it enhances the proliferation of pluripotent tendon cells and accelerates angiogenesis and hypercellularity in the tendon tissue16. In a rat tendon rupture model, administration of Sub-P was shown to enhance Achilles tendon healing and modulate sensory nerve plasticity, in terms of diameter of newly organised collagen and sensory nerve fibre ingrowth, respectively. Of note, the results indicated no difference between collagen fibre organisation and sensory nerve ingrowth after 6 weeks of treatment17.
Tendinosis does not only occur in physically active individuals, but also appear in people who conduct moderate physical activity. Besides, factors such as body weight, nutrition, and age may influence the physiological condition of tendon as well. Although the mechanism of development of tendinosis is yet to be fully discovered, it is evident that neural regulation plays a crucial role in tendon homeostasis. As one of the potent neuropeptides occurring in tendons, Sub-P is reported to influence both the process of tendon healing and progress of tendinosis. In view of the conflicting roles, previous comment suggested that the duration of Sub-P exposure determines its effects on the tendon, with prolonged Sub-P exposure leads to tendinopathic outcomes18. Of course, future works investigating the roles of Sub-P in the pathophysiology of tendon are highly desirable. Essentially, strategies on manipulating Sub-P levels would provide insight in the development of therapeutics combating tendon diseases.
References
1. Fong et al. J Orthop Res 2013; 31: 91-8. 2. Lake et al. Foot Ankle Clin 2009; 14: 663-74. 3. Millar et al. Nat. Rev. Rheumatol.2017; 13: 110-22. 4. Lopez. Clin Orthop Surg 2015; 7: 1. 5. Matthews et al. J Bone Jt Surg - Ser B 2006; 88: 489-95. 6. Tang et al. J. Orthop. Transl.2018; 14: 23-33. 7. Ackermann. Int. J. Exp. Pathol.2013; 94: 271-86. 8. Ackermann et al. Front Biosci 2009; 14: 5165-87. 9. Ackermann et al. Neuroreport 1999; 10: 2055-60. 10. Ackermann et al. J Orthop Res 2001; 19: 372-8. 11. Schizas et al. Scand J Med Sci Sport 2010; 20: 208-15. 12. Gargiulo et al. Neurol Res 2011; 33: 750-8. 13. Pradhan et al. Expert Rev. Mol. Med.2009; 11: e2. 14. Andersson et al. Br J Sports Med 2011; 45: 1017-22. 15. Pingel et al. Scand J Med Sci Sport 2013; 23: e353-60. 16. Zhou et al. J Musculoskelet Neuronal Interact 2014; 14: 349-58. 17. Carlsson et al. Scand J Med Sci Sport 2011; 21: 562-9. 18. Oh et al. Int J Mol Sci 2020; 21: 1-13.





