
Rabies is a zoonotic, and progressive neurological infection caused by Lyssavirus, affecting both animals and humans. Notably, more than 99% of human rabies are transmitted by unvaccinated dogs, and the disease affects more than 100 countries globally. Currently, there are rabies immune globulins (RIG) that are used as post-exposure prophylaxis (PEP) for individuals exposed to rabies. However, due to limit availability and safety concerns of RIGs (human RIG and equine RIG), a newer treatment approach utilising a mixture of two humanised monoclonal antibodies (mAbs) to provide a better coverage against different strains of rabies virus (RABV) has been considered. The aim of this article is to review the disease burden related to rabies, in addition to discuss the upcoming potential treatment which may help change the landscape of rabies and help countries achieve the “Zero by 30” target set by the World Health Organisation (WHO).
Rabies, a Silent Killer on the Loose
Rabies is a zoonotic, and progressive neurological infection caused by Lyssavirus, typically affecting all and warmblooded animals1. Remarkably, once clinical symptoms appear, rabies carries a 100% fatality rate2. Notably, more than 99% of human rabies case are transmitted via unvaccinated dogs2, affecting more than 100 countries globally, and rabies is slowly becoming the leading cause of death related to zoonotic aeitology3. Strikingly, rabies causes approximately 59,000 deaths and loss of more than 3.7 million disability adjusted life years (DALYs) annually, with most reported cases originating in rural areas of Africa and Asia3. One of the major contributing factors to this significant burden is the low availability of safe, affordable and effective biologics4. According to the WHO, early administration of immune globulins and vaccine remains an important aspect for the prevention of rabies by PEP (Figure 1)5.
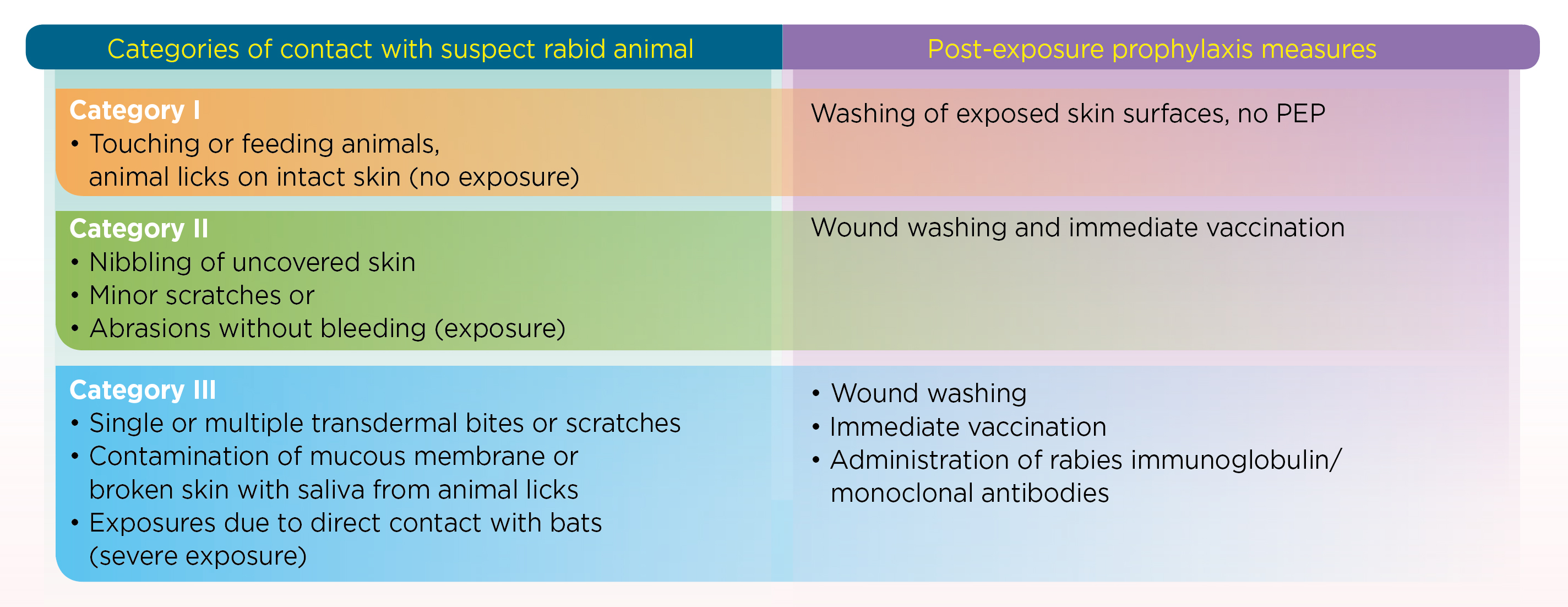
Figure 1. Category II and III exposures require human rabies vaccination5
Currently, PEP used against rabies include RIGs which are either prepared from humans (HRIG) or equine (ERIG) plasma. However, concerns are raised regarding their safety, immunogenicity, sustainability, and batch-to-batch variations, which had led to the pursual of a replacement to RIG4. Very recently, the WHO has recommended combining mAbs that target at least two non-overlapping epitopes on the RABV glycoprotein to overcome current RIG limitation, as well as maintaining the breadth of cross-reactivity necessary for effective protection4. This has ushered a new treatment era with the introduction of SYN023*, which is a mixture of two humanised monoclonal IgG kappa antibodies, CTB011 and CTB012, that bind to non-overlapping epitopes within highly conserved regions of the RABV outer enveloped glycoprotein6.
Unleashing the Power of Monoclonal Antibodies
The humanised mAbs are an ideal alternative to RIG since they are more affordable, accessible, and standardised7. Even though there are currently five mAbs that has been tested in clinical trials, not all mAbs have equal neutralising power. For instance, the first mAb was approved by the Indian Regulatory Agency in 2016 against RABV, however, it has shown to be ineffective in neutralising a rare rabies variant found in the Peruvian Bats8. Why? It may be due to the fact that the formulation only contains one type of mAb7, therefore, deemed ineffective against other strains of the virus. On contrary, the SYN023*, a mixture of two anti-rabies humanised IgG1 kappa mAbs may help overcome the shortfalls of other ineffective mAbs currently available in the market since SYN023* is able to neutralise more than 15 contemporary clinically isolated stains of rabies collected from China and 10 predominant strains in the United States (US)7. The effectiveness of SYN023* was evaluated in a phase 2b randomised controlled trial (RCT) by Quiambao et al., (2024). 448 patients in two risk substrata of WHO Category III exposure were randomised to receive either 0.3 mg/kg SYN023* or 0.133 mL/kg HRIG injected in and around the wound site(s) plus a course of rabies vaccination. Patients were followed for safety and absence of rabies for ≥1 year.
Remarkably, the geometric mean titer (GMT) for serum rabies virus neutralising activity (RVNA) was higher with the SYN023* throughout 2-weeks post-treatment period. Strikingly, 99.4% of SYN023* recipients had proactive RVNA levels on Day 4 compared to only 4.5% of HRIG recipients. These differences widened even further on the day 8 with 98.1% of SYN023* recipients were protected against RABV compared to only 12.2% of HRIG recipients. These findings support the notion that the anti-rabies immune response with SYN023* was non-inferior to HRIG since the SYN023*: HRIG ratio of RVNA GMTs at day 8 was 19.42, exceeding the 10% superiority margin (p<0.0001) (Figure 2)6.
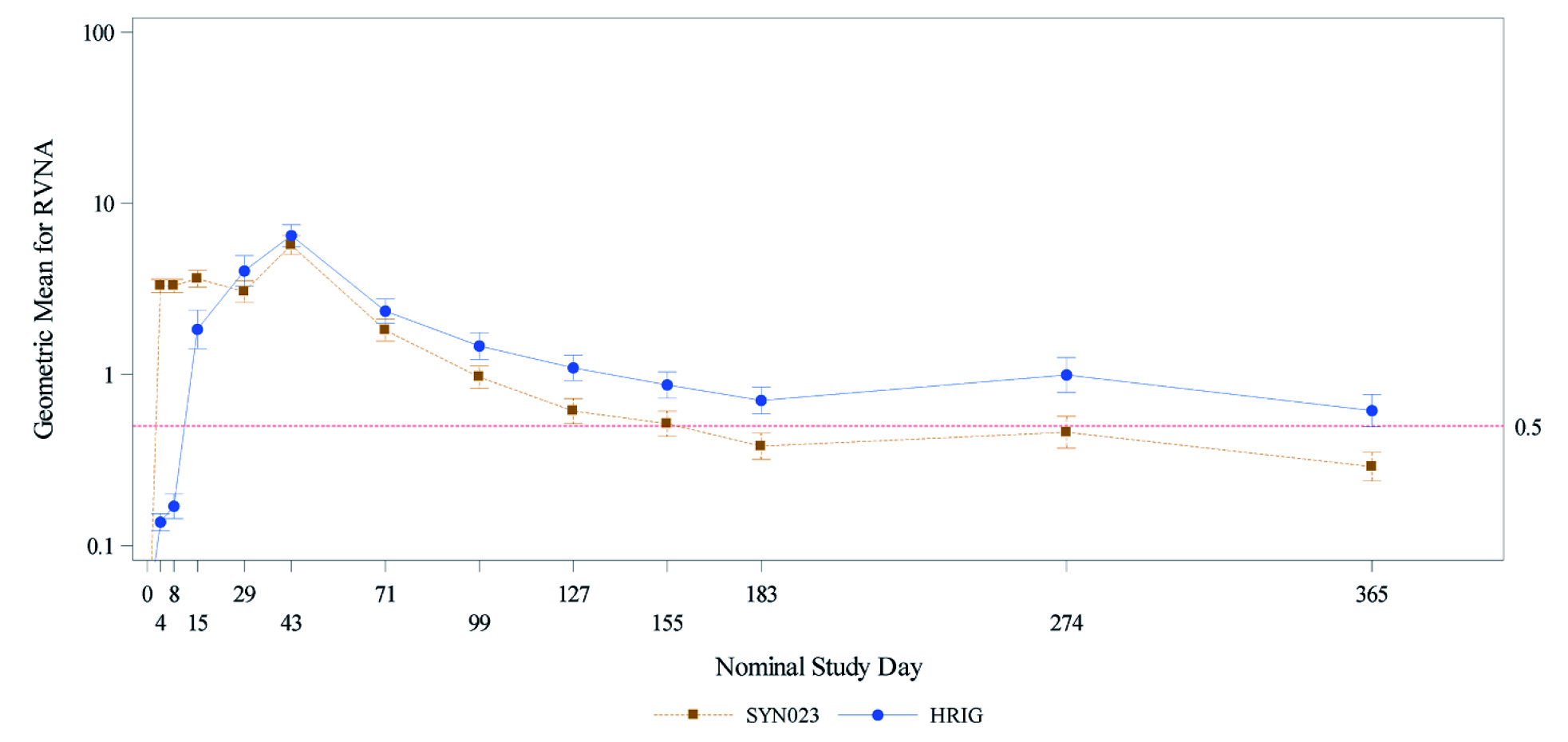
Figure 2. Geometric Mean Rabies Virus Neutralising Activity over the study period- Normal risk group per protocol population error bars denotes 95% confidence interval. Rabies virus neutralising activity (RVNA) values less than the assay lower limit of quantitation (LLoQ) were set to LLoQ/2 (0.05 IU/mL) for geometric mean RVNA calculation6. HRIG= human-derived rabies immunoglobulin, RVNA= rabies virus neutralising activity.
Here, SYN023* demonstrated its ability to provide a higher RVNA than HRIG soon after rabies exposure, especially at WHO Category III exposure6. In the wake of rising rabies cases globally, especially after the coronavirus-2019 (COVID-19) pandemic, the four world bodies, namely the WHO, the Food and Agriculture Organisation (FAO), the World Organisation for Animal Health (OIE), and the Global Alliance for Rabies Control (GARC) endorsed forming a global support system to eradicate the disease by the year 2030 (Zero by 30)9. This framework calls for extending the vaccination of dogs to reduce the risk of human rabies, in addition to curb the global rising rabies cases with a better disease management9. In conclusion, rabies is a deadly infectious disease that targets different organs, particularly the brain and the lungs10. Considering dog bites being the main source of transmission of this disease, appropriate administration of anti-rabies vaccines and immune globulins remains vital for rabies prevention and eventual eradication.
Footnote
*SYN023 is currently not approved by the European Medical Agency (EMA) and Food and Drug Administration (FDA). It is only approved for clinical trials by the Chinese Food and Drug
Administration (CFDA) in June 20177.
References
1. Singh R, et al. Vet Q 2017; 37(1): 212-51. 2. Jane Ling MY, et al. BMJ Open 2023; 13(5): e066587. 3. Jiang X, et al. One Health 2024; 18: 100743. 4. Chao TY, et al. PLoS Negl Trop Dis 2017; 11(12):
e0006133. 5. Organization WH. https://www.who.int/news-room/fact-sheets/detail/rabies (accessed 12/9/2024 2024). 6. Quiambao BP, et al. Vaccine 2024; 42(22): 126018. 7. Ding Y, et al.
Antiviral Research 2020; 184: 104956. 8. Fan L, et al. Hum Vaccin Immunother 2022; 18(1): 2026713. 9. Pattnaik P, et al. Cureus 2023; 15(12): e50424. 10. Khairullah AR, et al. Open Vet J 2023;
13(11): 1385-99.





