
Doctor at the National Health Services (NHS), United Kingdom (UK)
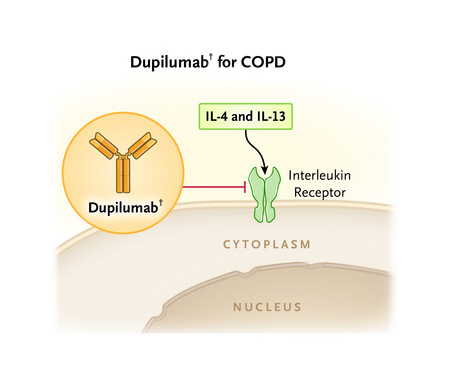
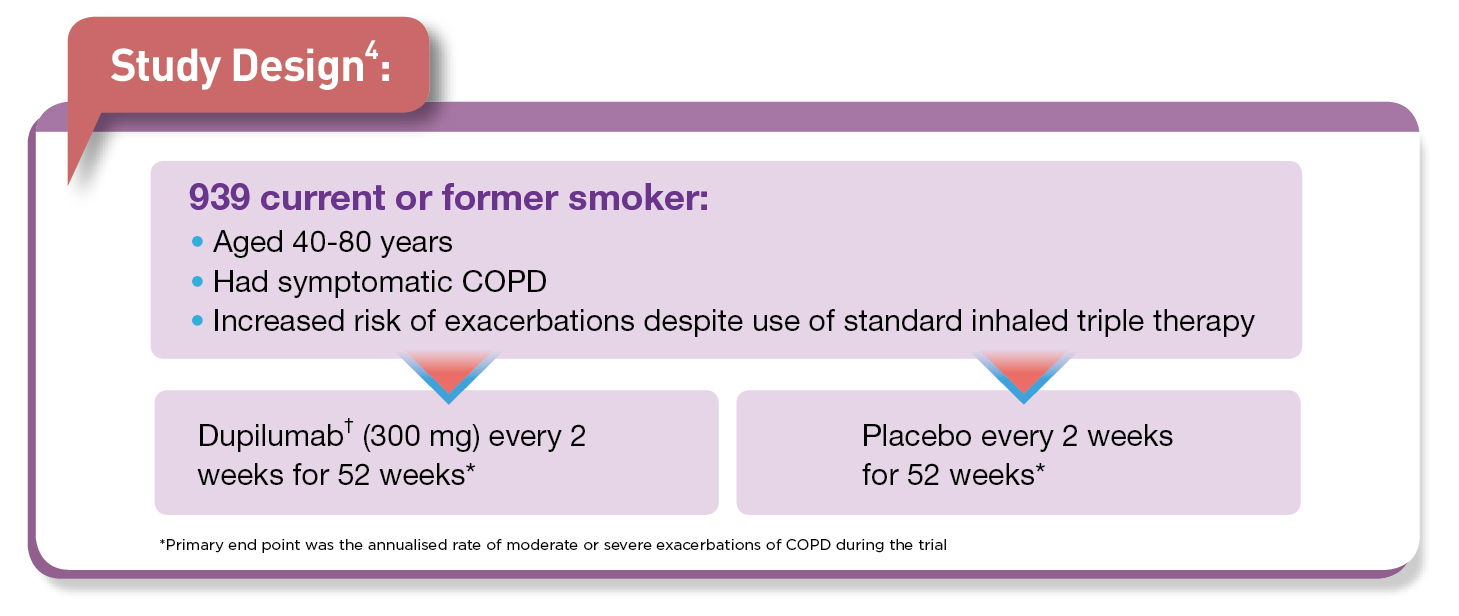
Chronic obstructive pulmonary disease (COPD) is an umbrella term for a variety of lung conditions and growing evidence indicates that eosinophilic inflammation1 is involved 10-40% of patients with COPD2. Notably, COPD is one of the top three cause of death, globally with 212.3 million prevalent cases and 3.3 million deaths reported globally in 20191. Moreover, the unmet treatment needs persist since COPD is often underdiagnosed, and undertreated3. Interestingly, in some patients with COPD, type 2 inflammation may increase exacerbation risk and recent study by Bhatt et al., (2023) evaluated the efficacy and safety of dupilumab† in COPD patients with blood eosinophil count of ≥300 per microlitres4.
Conclusion4:
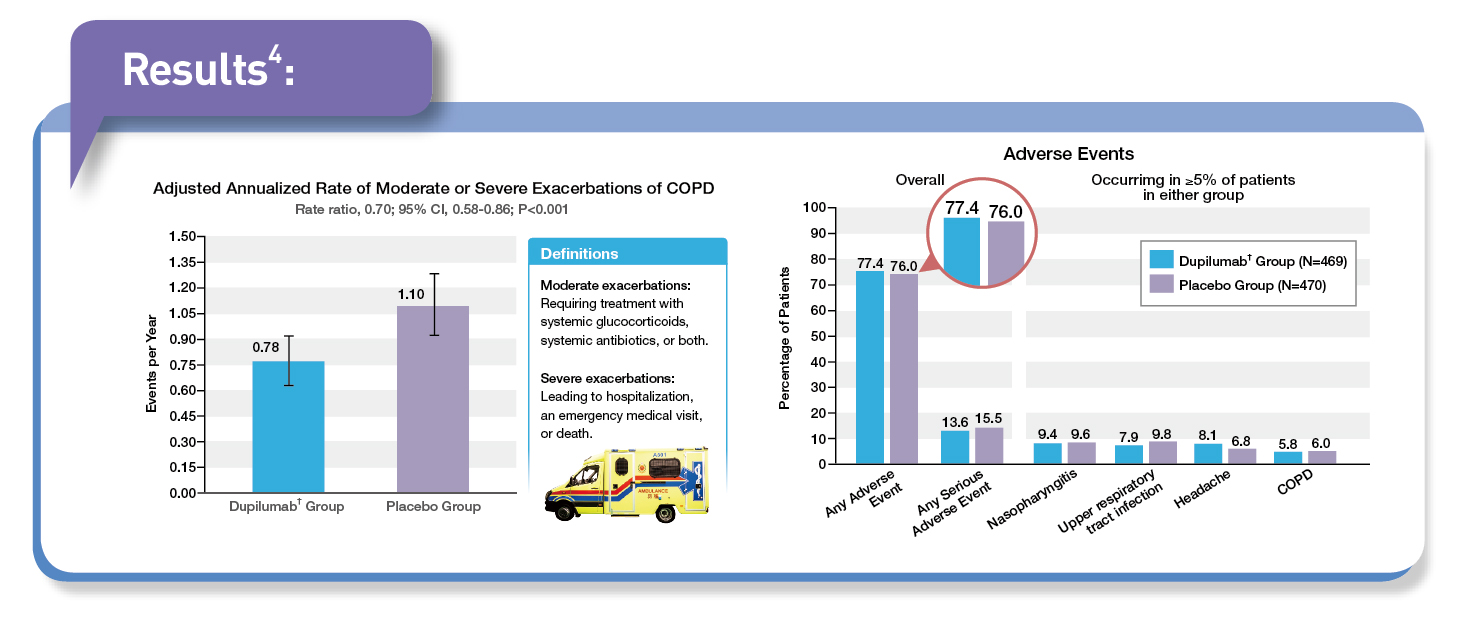
In patients with COPD who had type 2 inflammation as indicated by elevated eosinophil counts, add-on treatment with dupilumab† resulted in a lower annualised rate of moderate or severe exacerbations than placebo.
† Dupilumab is currently pending Federal Drug and Administration (FDA) priority review as an add-on maintenance treatment in adult patients with uncontrolled COPD associated with a history of exacerbations and an eosinophilic phenotype5
References
1. Ko FWS, et al. Respiratory Medicine 2024; 222: 107516. 2. Borish L, et al. Annals of Allergy, Asthma & Immunology 2023; 130(5): 617-21.e1. 3. Ozoh OB, et al. Am J Respir Crit Care Med 2023; 208(4): 352-4.
4. Bhatt SP, et al. New England Journal of Medicine 2023; 389(3): 205-14. 5. Phalguni D. Pharmaceutical Techology. 23 Feb. 2024, www.pharmaceutical-technology.com/news/fda-to-decide-on-sanofi-regenerons-dupixent-for-copd-in-june/?cf-view. Accessed 29 May 2024.





