
Heart failure (HF) is a complex clinical syndrome that results from a functional or structural heart disorder impairing the ventricular filling or ejection of blood to the systemic circulation1. It affects more than 64 million people worldwide and remains a significant global public health burden. In fact, the burden of HF on health care expenditures worldwide is concerning as the total cost of HF was estimated to be 30.7 billion US dollars (USD) in the US alone in 2012, with a projection of 127% increase by the year 20302. HF is classified based on symptoms and calculated left ventricular ejection fraction (LVEF). HF due to left ventricular dysfunction is categorised into HF with reduced ejection fraction (HFrEF), HF with preserved ejection fraction (HFpEF) and HF with mid-range ejection fraction (HFmrEF). HFrEF is defined as an ejection fraction (EF) of less than 40%, whereas HFpEF is defined as HF with an EF greater than 50%. Contrary to this, HFmrEF is defined as HF with an EF of 40% to 50%1.
Recent epidemiological data suggested an increasing prevalence of HFpEF affecting more than half of patients with HF. Moreover, HFpEF carries significant morbidity and mortality since no treatment has been shown to improve the outcomes of HFpEF, in contrast to the efficacy of treatment in HFrEF3. Interestingly, HFpEF is often under-recognised and results in substantial resource utilisation. Therefore, the American College of Cardiology (ACC) have complied an expert consensus to help guide clinicians on the management of HFpEF.
The prevalence of HF with HFrEF and HFpEF continues to rise globally despite current advances in diagnostic and improvements to medical management4. Currently, there are several challenges faced by clinicians in diagnosing and treating patients with HFpEF. The diagnostic challenge with HFpEF remains significant since there are no single tests available to help establish the diagnosis5. Moreover, since the signs and symptoms of HF are similar to non-cardiac diseases, therefore, the diagnosis of HF can easily be missed. In fact, the rate of HF misdiagnosis ranged from 16.1% in the hospital setting to 68.5% when referred by the general practitioner to the specialist6. In view of this, the ACC has advocated considering both non-cardiac and cardiac causes that may be present with signs of congestion/ and or symptoms of dyspnoea, exercise intolerance, or congestion with preserved EF since misdiagnosis may result in a missed opportunity to institute an effective disease-directed therapy5.
The challenges in the diagnosis and management of HFpEF are even more pertinent in females compared to males since females have a higher ejection fraction (EF) with more preserved left ventricular (LV) global longitudinal strain. As a result, they are less likely to develop reduced EF and echocardiographic imaging in females with HFpEF are more likely to show significant concentric LV remodelling with impaired LV relaxation and higher diastolic stiffness compared to males with HFpEF5. Aside from diagnostic challenges and gender discrepancies, the prognosis and survival of individuals with HFpEF are poor, with nearly 40% of HFpEF patients dying within 5 years following discharge from hospital for HF7. Therefore, due to the challenges faced in diagnosing and managing HFpEF, the ACC recommended that primary care clinicians should be aware of HFpEF in their differential diagnosis of dyspnoea, exercise intolerance and oedema. Moreover, they should recognise when a cardiology referral may be useful; contrary to this, the cardiology specialist should exclude the presence of an alternative diagnosis and identify a referral to a HF specialist who may then pursue advance testing in cases of the diagnostic dilemma5.
The universal definition of HF may provide a useful guide for clinicians during the diagnosis of HF. Nevertheless, a diagnosis of HFpEF may be more difficult given that the echocardiogram may not demonstrate obvious structural or functional cardiac abnormalities and the natriuretic peptide level may be normal, especially among obese individuals5. To help aid in the diagnostic evaluation of suspected HFpEF, the use of a clinical scoring system may be useful, according to the ACC expert consensus. The two common scoring systems used are H2FPEF and HFA-PEFF. The H2FPEF is a more practical system consisting of 6 components, while the HFA-PEFF algorithm utilises 4 steps as highlighted in Figure 15.
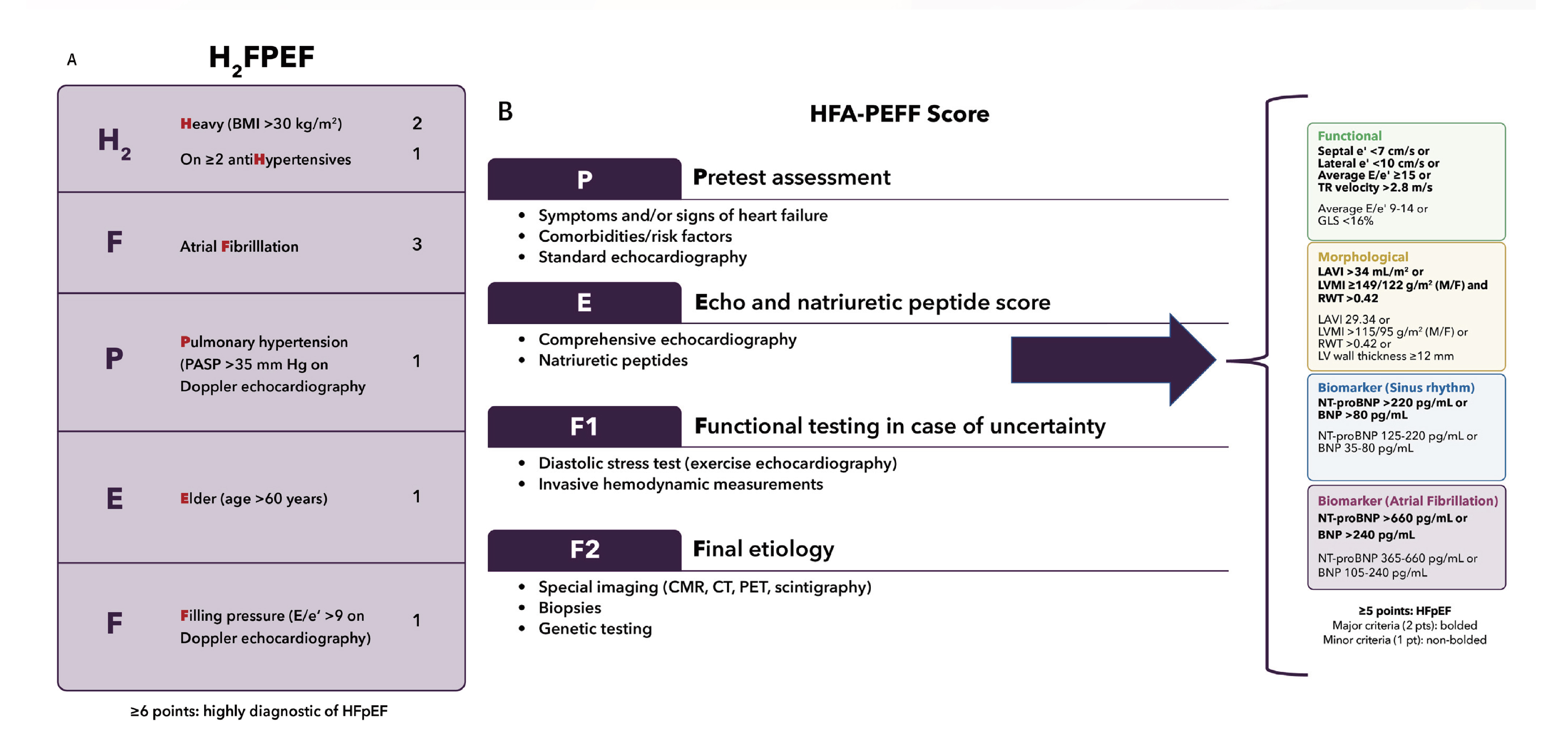
Figure 1. (A) The H2FPEF score includes 6 clinical accessible factors. (B) The Heart Failure Association (HFA)-PEFF includes a more diagnostic algorithm starting with pretest assessment, echocardiographic and natriuretic peptide score, functional testing for an advanced evaluation, and final aetiology assessment5.
Both the H2FPEF and HFA-PEFF scores can aid clinicians in diagnosing HFpEF; nonetheless, they have their own limitations. For instance, a low H2FPEF score in the context of HF symptoms and signs should not be used to exclude the HFpEF diagnosis. Furthermore, gender discrepancy remains an important issue during the diagnostic process, according to the ACC. For instance, echocardiographic imaging in females with HFpEF may underestimate LV dysfunction, especially in females with LVEF of 50% to 55%, since these LVEF values may actually be abnormal in females5. Therefore, those with evidence of congestion and preserved EF should undergo a further diagnostic evaluation to assess for comorbidities such as hypertension, obesity, atrial fibrillation (AF) and chronic kidney disease (CKD), with HFpEF being a diagnosis of exclusion according to the 2023 ACC expert consensus5.
Medical therapy for HFpEF is often discouraging since the array of negative trials with no demonstrated benefits seen in patients with HFpEF in the past5. However, recent clinical trials have demonstrated the benefit of GDMT in individuals with HFpEF since it helps improve symptoms, functional capacity, as well as reduces the morbidity and mortality associated with HF5. In this aspect, the sodium-glucose cotransporter-2 inhibitors (SGLT-2is), particularly dapagliflozin, have been evaluated in a multicenter randomised controlled trial (RCT) in patients with HF and HFpEF. It has been shown to improve the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CS), which is a measure of heart failure-related health status as early as 12 weeks after initiating treatment. In addition, the adverse events were comparable to that of placebo8.
Therefore, the 2023 ACC expert consensus has advocated the use of SGLT-2is in patients with HF since SGLT-2is have shown to reduce the risk of hospitalisation for HF (HHF) and cardiovascular death across all EF subgroups as demonstrated in the DELIVER (Dapagliflozin Evaluation to improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure) trial with 6,263 patients with HF and LVEF of >40% receiving either 10 mg of dapagliflozin once daily or matching placebo, in addition to their usual therapy5,9. The trial concluded that cardiovascular deaths and first and recurrent worsening HF events were lower in the dapagliflozin group than in the placebo group as illustrated in Figure 29.
 -3.jpg)
Figure 2. Dapagliflozin reduces worsening heart failure events compared to placebo9.
In addition, the post-hoc analysis from DELIVER trial showed dapagliflozin was safe and improved outcomes irrespective of baseline N-terminal pro B-type natriuretic peptide (NT-proBNP) concentrations in HFmrEF or HFpEF, with the greatest benefit seen in patients with higher NT-proBNP concentrations10. Therefore, in view of these findings, the 2023 ACC expert consensus has advocated SGLT2is use in all HFpEF patients without contraindications, ideally once stable during hospitaliation for index event5. In conclusion, given the complexity of HFpEF, a multidisciplinary approach with evidence and guideline-based therapies should be considered.
References
1. Malik A, Brito D, Vaqar S, Chhabra L. Congestive Heart Failure. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023. 2. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovascular Research 2022; 118(17): 3272-87. 3. Ilieșiu AM, Hodorogea AS. Treatment of Heart Failure with Preserved Ejection Fraction. In: Islam MS, ed. Heart Failure: From Research to Clinical Practice: Volume 3. Cham: Springer International Publishing; 2018: 67-87. 4. Sullivan RD, Gladysheva IP. Advances and Challenges in Diagnosis and Management of Heart Failure. Diagnostics (Basel) 2022; 12(5). 5. Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology 2023; 81(18): 1835-78. 6. Wong CW, Tafuro J, Azam Z, et al. Misdiagnosis of Heart Failure: A Systematic Review of the Literature. J Card Fail 2021; 27(9): 925-33. 7. Hossain MZ, Chew-Graham CA, Sowden E, et al. Challenges in the management of people with heart failure with preserved ejection fraction (HFpEF) in primary care: A qualitative study of general practitioner perspectives. Chronic Illn 2022; 18(2): 410-25. 8. Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med 2021; 27(11): 1954-60. 9. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. New England Journal of Medicine 2022; 387(12): 1089-98. 10. Myhre PL, Vaduganathan M, Claggett BL, et al. Influence of NT-proBNP on Efficacy of Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction. JACC Heart Fail 2022; 10(12): 902-13.





