
The Role of Senescence in Age-related Cardiovascular Diseases and Its Potential Solution
According to World Health Organization, cardiovascular diseases (CVDs) are one of the major causes of death globally, responsible for 17.9 million deaths every year1. It has been suggested that cellular senescence is associated with a pro-inflammatory state, which contributes to age-related tissue and organ function impairment. In particular, senescence of endothelial and cardiac cells appears to be accelerated in age-related CVDs, suggesting the pivotal role of senescence in CVD development2. Essentially, clearance of senescent cells was found to alleviate CVDs phenotypes, thereby opened up opportunities for treating and preventing CVDs3. Senolytics, a class of drugs that selectively eliminates senescent cells, has thus emerged as a potential cardioprotective candidate4.
Senescence and Increased Risk for CVDs
Senescence, the cellular driver of aging, is defined as the irreversible replicative arrest of cells accompanied by a pro-inflammatory secretory phenotype and may be induced by telomere shortening or by stress5. While some senescent cells facilitate tissue repair and are cleared by the innate immune system after injury resolution, studies typically found senescent cells to be anti-apoptotic and accumulate in tissue6. Their resistance to apoptosis was attributed to the up-regulation of the senescent cell anti-apoptotic pathways (SCAPs) related to molecules such as BCL-XL and dependence receptors7. Senescent cells are also characterised by the exhibition of the senescent-associated secretory phenotype (SASP). In most senescent cells, elements of SASP include IL-1α, IL-1β, IL-6, IL-8, IL-18, and TNF-α, all of which increase the risk for CVDs and promote senescence locally8.
Senescence in several cell types in the cardiovascular system, commonly identified by the presence of the senescence-associated marker p16LINK4a, has been associated with CVDs9. In the vasculature, senescent endothelial cells, likely induced by telomeric shortening, are found to accumulate in human atherosclerotic lesions and are considered one of the drivers of atherogenesis10. Senescence in endothelial cells may directly disrupt cellular proliferation, permeability, and motility, thereby increasing the chance of endothelial erosion and intra-plaque haemorrhage associated with plaque rupture9. Meanwhile, senescent vascular smooth muscle cells (VSMCs) have been associated with necrotic core enlargement and plaque calcification in atherosclerosis. They also secrete various matrix metalloproteinases (MMPs), which leads to cap thinning and, subsequently, plaque destabilisation9. Hence, senescent vasculature cells have been associated with plaque progression.
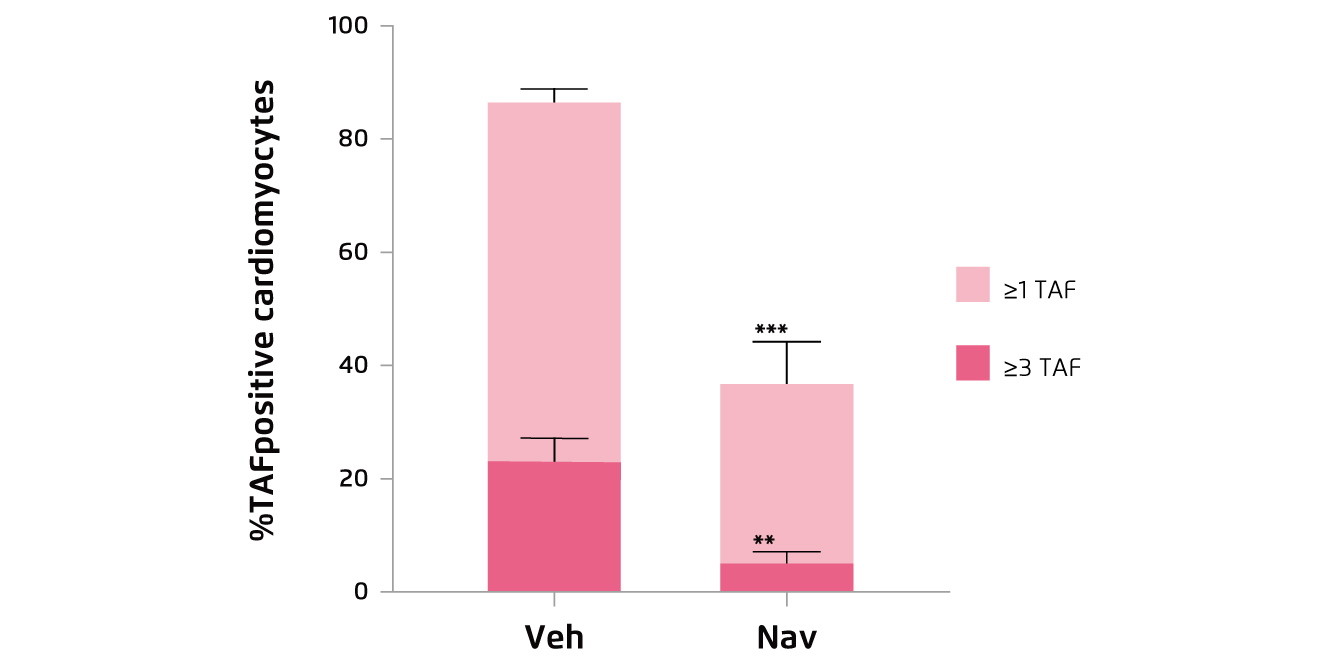
Figure 1.
Percentage of TAF-positive CMs in aged mice treated with vehicle (Veh) or navitoclax (Nav)16
While senescent VSMCs display the canonic SASP, senescent cardiomyocytes in the heart can display a noncanonical senescence-associated secretory phenotype (SASP) that is characterised by the enhanced expression of Edn3, TGFβ2, and Gdf1511. This SASP was found to be antiproliferative, pro-fibrotic, and pro-hypertrophic in vitro, and was suggested to induce chronic sterile inflammation, heart remodelling, as well as render other normal cells senescent12. With the influence of the noncanonical SASP, cardiomyocytes increase in size and cause a significant increase in myocardial thickness, especially in the left ventricle13. This aging-related left ventricular hypertrophy, albeit within normal limits, is associated with a retarded early diastolic cardiac filling, increased cardiac filling pressure and hence a higher chance of heart failure with relatively normal systolic function13. Besides, senescence of cardiac progenitor cells (CPCs) was reported to reduce the potency of CPCs and, hence, hinder cardiac tissue regeneration after cardiovascular events14. Thus, senescence of cells in cardiovascular tissues would adversely affect the maintenance and regeneration of the tissue.
Selective Clearance of Senescent Cells by Senolytics
In light of the adverse effects of senescent cells on the cardiovascular system, eliminating senescent cells may help prevent CVDs or improve their prognosis. Senolytics is a class of drugs that eliminates only senescent cells and has no effect on non-senescent cells4. Its selectivity is made possible by the resistance to apoptosis observed in senescent cells, which was attributed to the up-regulation of the senescent cell anti-apoptotic pathways (SCAPs)7. SCAPs related to BCL-2/BCL-XL, PI3K/AKT, p53/p21/serpine, dependence receptors/tyrosine kinases, and HIF-1α have been identified, thereby creating opportunities for the development of senolytics targeting these pathways7. Navitoclax (ABT-263), for instance, is a well-studied senolytic agent targeting BCL-XL and is senolytic in HUVECs and human lung fibroblast cell strain IMR-9015.
Evidence on the Cardioprotective Effects of Senolytics
The cardioprotective effects have been demonstrated in former animal trials. For instance, in the study by Anderson et al. (2019), aged wild-type mice were treated with either vehicle or navitoclax intermittently for 2 weeks. The results indicated that navitoclax significantly reduced cardiomyocytes containing dysfunctional telomeres, as reflected by telomere-associated DNA damage response foci (TAF, Figure 1), and attenuates cardiac hypertrophy and fibrosis as compared to the control16. Likewise, in another aged mice model by Walaszczyk et al. (2019), 2 cycles of 7-day treatment with navitoclax was demonstrated to effectively clear p16-positive senescent cells from the hearts of the treated mice (Figure 2)17. Furthermore, the results showed that navitoclax treatment significantly reduced the age-dependent increase in left ventricular mass but did not impact on ejection fraction17. The findings in previous works thus suggested that senescent cells are major contributors to impaired cardiac function, whereas senolytics would be potential candidates combating the condition.
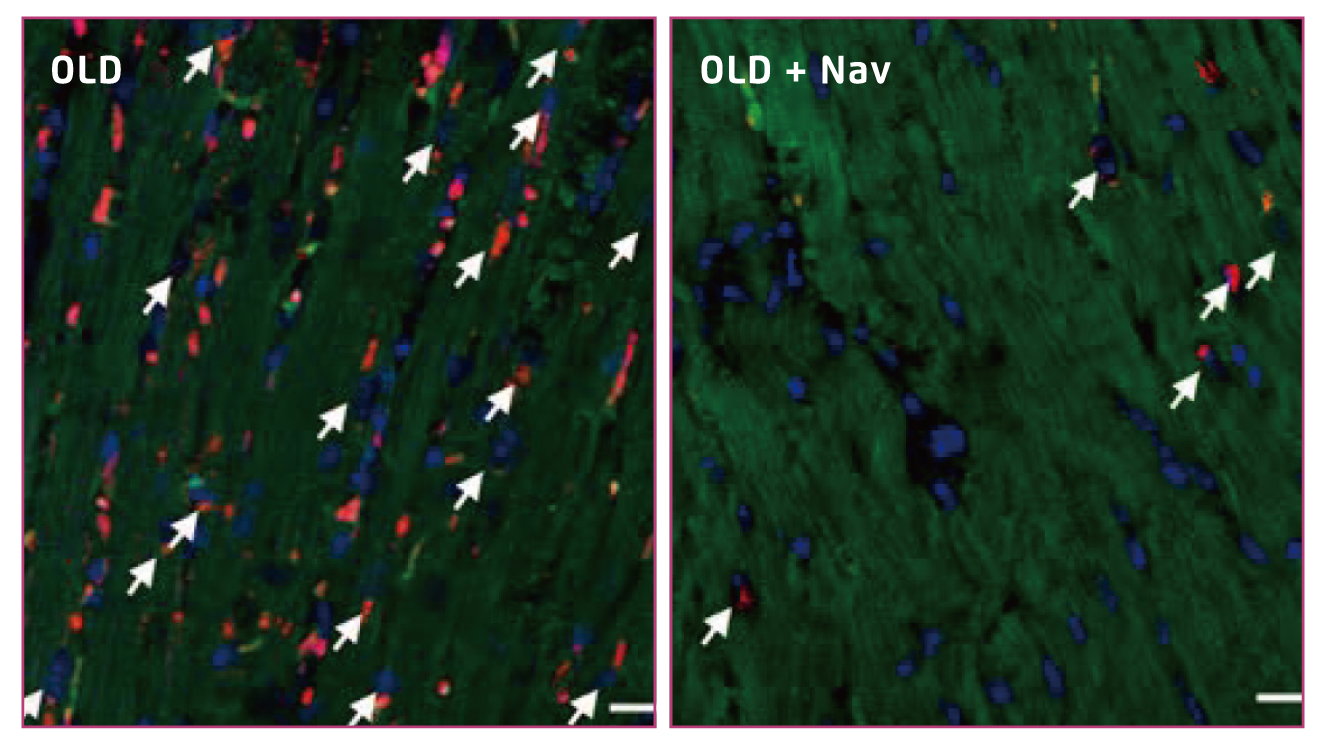 Figure 2.
Figure 2.
Clearance of senescent cardiomyocytes (indicated by arrows)17
Future Perspectives
With the growing proportion of elderly population, the disease burden of CVDs becomes increasingly significant. Previous investigations visualised the pivotal role of senescent cardiomyocytes in the development of aged-associated CVDs and demonstrated the therapeutic potential of senolytics in controlling the diseases. Nonetheless, senescence in cell types other than cardiomyocytes in the cardiovascular system can also contribute to the development of CVDs and may also be targeted by senolytics to protect against CVDs. Hence, further works on the pathological mechanism of senescent cells-associated CVDs, as well as other aged-related diseases, are essential for the development of new therapeutic senolytics.
Acknowledgement
The author appreciates the editorial support by Ms Zoe Tsz Yan Wan of the School of Biomedical Sciences, CUHK in the preparation of this article.
References
1. World Health Organization.Cardiovascular diseases. 2021. 2. Olivieri et al. Curr Pharm Des 2013; 19: 1710-9. 3. Childs et al. J Clin Invest 2018; 128: 1217-28. 4. Amaya-Montoya et al. Adv Ther 2020; 37: 1407-24. 5. Van Deursen. Nature.2014; 509: 439-46. 6. Childs et al. EMBO Rep 2014; 15: 1139-53. 7. Kirkland et al. EBioMedicine 2017; 21: 21-8. 8. ReaI et al. Front Immunol 2018; 9: 1. 9. Shimizu et al. J Cardiol 2019; 74: 313-9. 10. Minamino et al. Circulation 2002; 105: 1541-4. 11. Von Zglinicki et al. Antioxidants Redox Signal 2021; 34: 308-23. 12. Wagner et al. J Mol Cell Cardiol 2020; 138: 136-46. 13. Strait et al. Hea rt Fail Clin 2012; 8: 143-64. 14. Lewis-McDougall et al. Aging Cell 2019; 18: e12931. 15. Zhu et al. Aging Cell 2016; 15: 428-35. 16. Anderson et al. EMBO J 2019; 38. DOI:10.15252/embj.2018100492. 17. Walaszczy et al. Aging Cell 2019; 18. DOI:10.1111/acel.12945.





