

Editor-in-Chief, V·Pulse

MD, DSc
Rheumatoid arthritis (RA) is a chronic inflammatory disease that can create joint damage and bone destruction, causing severe pain and disability. The disease also manifests as extra-articular disease, that can affect most organs of the body, with a reduced life expectancy1. Tight disease control based on the treat-to-target concept, earlier disease diagnosis, and an expansion of more efficient RA treatments, including biological disease-modifying antirheumatic drug (bDMARD), currently result in, about 50% of patients with early RA achieving sustained remission2. Notably, international guidelines for the management of RA suggest tapering bDMARDs in patients in persistent remission3. Because of occurrence of RA flares during the tapering process, accurate prediction of RA flare risk guide clinical decision making to minimise the number of flares while maintaining most of the bDMARD dose reduction. Remarkably, reliable models based on machine-learning (ML) algorithms could be helpful tools for individual flare prediction and optimise RA management.
W hen RA patients have well-controlled disease, taper medication should be considered to reduce treatment cost and prevent long-term side effects4. The guidelines of the European League Against Rheumatism (EULAR) on the management of RA recommend tapering in patients with persistent remission. Current literature recommends “disease activity-guided dose optimisation” (DGDO) as a cost-effective approach for tapering. The DGDO approach refers to the gradual tapering of dose, usually by increasing the administration interval, until either disease activity flares or the biological disease-modifying antirheumatic drug (bDMARD) is discontinued3.
The randomised controlled trial (RCT) by van Herwaarden et al. (2015) demonstrated that 63–80% of RA patients could taper or even stop their bDMARD with DGDO5. Nonetheless, given the trial-and-error nature of DGDO, flares occur occasionally during the tapering process, even though the impact of the short flares on radiographic progression or long-term disease activity is considered modest3. Accordingly, accurate prediction on the risk of RA flares could potentially guide whether a bDMARD can be tapered in a particular patient without flare.
Conventionally, the risk of RA flares is evaluated on the occurrence of risk factors. For instance, in the multivariate analysis of real-world data from a cohort of 199 RA patients by Chaiamnuay et al. (2022), positive anticitrullinated peptide antibodies (ACPA) or rheumatoid factor (RF) (hazard ratio [HR]: 1.949, p=0.039, Figure 1A), time to DMARDs commencement (HR: 1.017, p=0.013), time to remission/low disease activity (LDA, HR: 1.037, p=0.001), tapering DMARDs promptly when persistent remission/LDA was achieved (HR: 2.497, p=0.001, Figure 1B), and the higher disease activity score 28 (DAS28) when remission/LDA was achieved (HR: 2.589, p=0.004) were identified as the predictors of RA flares6.
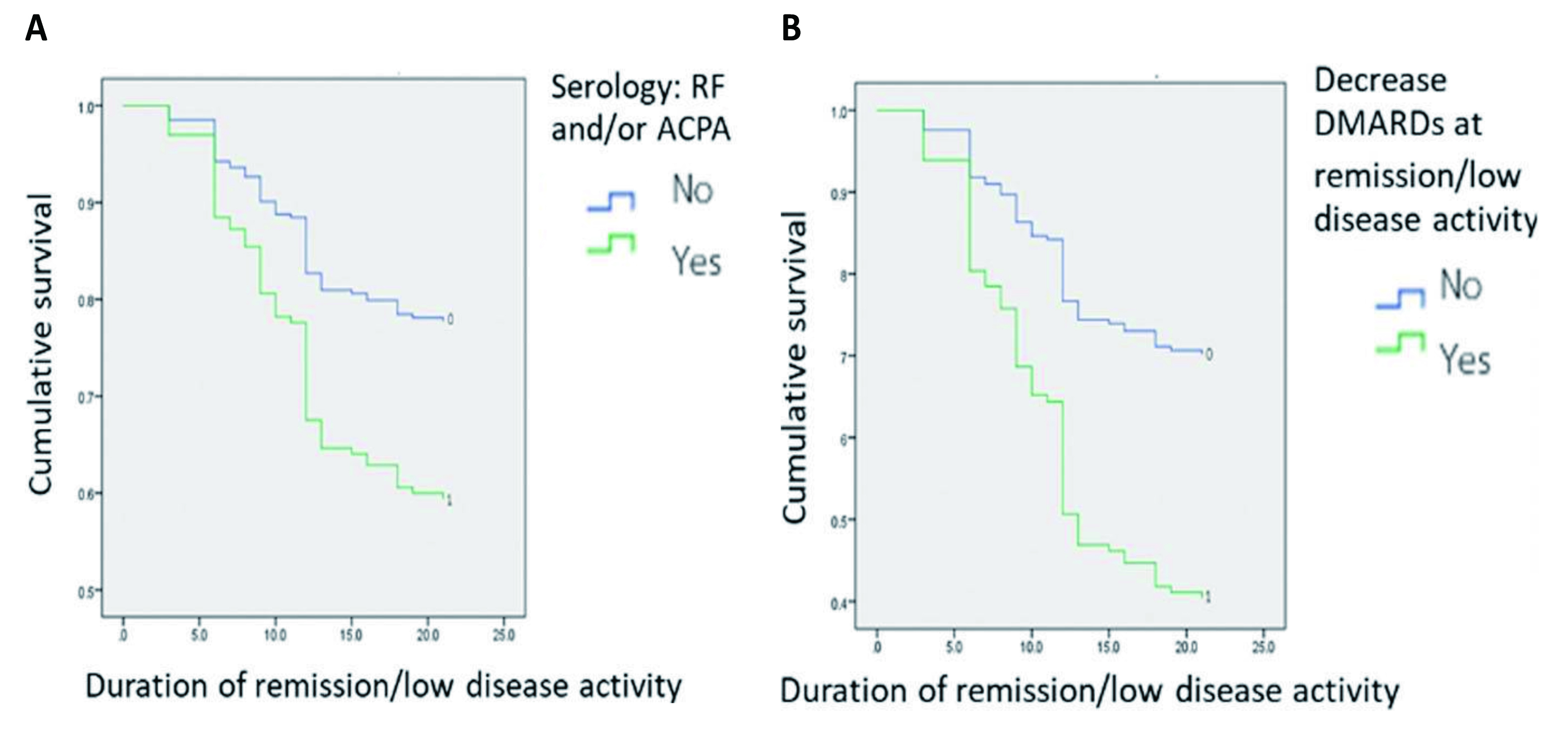
Figure 1. Kaplan–Meier survival curves for cumulative sustained remission/LDA rates stratified by A) occurrence of RF/ACPA, and B) tapering DMARDs at remission/LDA6
Although various risk factors of RA have been identified in previous investigations, their positive predictive values for the disease are suboptimal7; artificial intelligence (AI) could assist in this instance. AI is defined as the capability of a machine to imitate intelligent human behaviour; the potential applications of AI in various medical fields, including rheumatology, have been explored extensively, e.g., image processing in pathology and drug discovery. ML is a subset of AI in which intelligence is acquired through practice, and the goal of ML is to make accurate predictions with less focus on inference8.
The practical implementation of AI-powered analysis in rheumatology was illustrated in a recent study by Chung et al. (2021) in which ML models for the genomic prediction of RA and systemic lupus erythematosus (SLE) were established. The study included genome-wide single nucleotide polymorphism (SNP) data of 2,094 RA patients and 2,190 SLE patients obtained using the Taiwan Biobank version 2 array. The ML methods used were logistic regression, random forest, support vector machine, gradient tree boosting, and extreme gradient boosting. Human leukocyte antigen (HLA) imputation was performed using the HLA Genotype Imputation. The results suggested that, compared to logistic regression, all the 4 methods showed significantly better prediction performance (Figure 2). The results further indicated that genetic variations at HLA-DQA1, HLA-DQB1, and HLA-DRB1 were crucial for differentiating RA from SLE9. Nonetheless, subsequent verification is required to confirm the accuracy of the ML models.
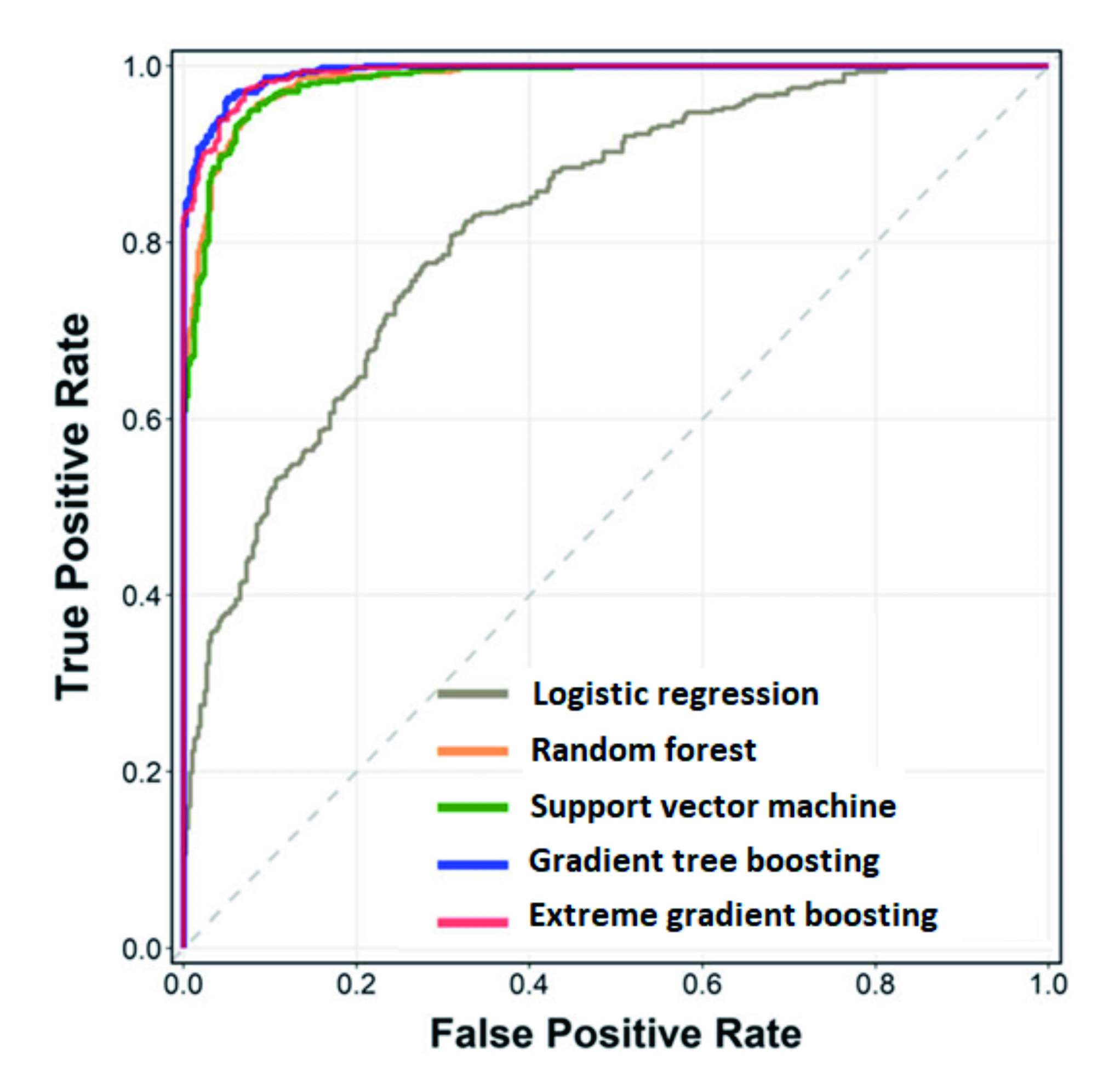
Figure 2. ROC curve on ML model prediction performance9
According to the treat-to-target approach, escalating therapy in RA patients with moderate and high disease activity is advisable to achieve rapid disease control and prevent structural damage as well as the associated functional limitations. Once remission is achieved, tapering medication should be considered. As noted, tapering may minimise side effects and reduce drug burden. However, the process can also increase the risk of flares10. In clinical practice, patients and rheumatologists have little support in making the best treatment decision. Considering this situation, Labinsky et al. (2022) conducted a study to include an AI-powered flare risk prediction tool to support the management of RA.
Longitudinal clinical routine data of RA patients were used to develop and test the AI-powered clinical decision support systems (CDSS). 5 physicians were asked to assess 10 patients' flare risk, to provide a treatment decision, and to assess their decision confidence with and without access to the CDSS for predicting flare risk. CDSS usability and acceptance were assessed using the system usability scale (SUS) and net promoter score (NPS)11.
The results demonstrated that the flare prediction tool reached a sensitivity of 72%, a specificity of 76%, and an area under the curve (AUC) of 0.80 (Figure 3). Interestingly, the perceived flare risk and treatment decisions varied largely between physicians. Applying the CDSS was reported to increase tapering decisions compared to the original decisions by the treating physicians (50% vs 20%). Remarkably, CDSS reduced deviations between physicians and the prediction tool. Besides, the AI-powered tool was reported to be well-accepted by physicians11. These findings suggest that CDSS could support physicians by decreasing assessment deviations and increasing treatment decision confidence.
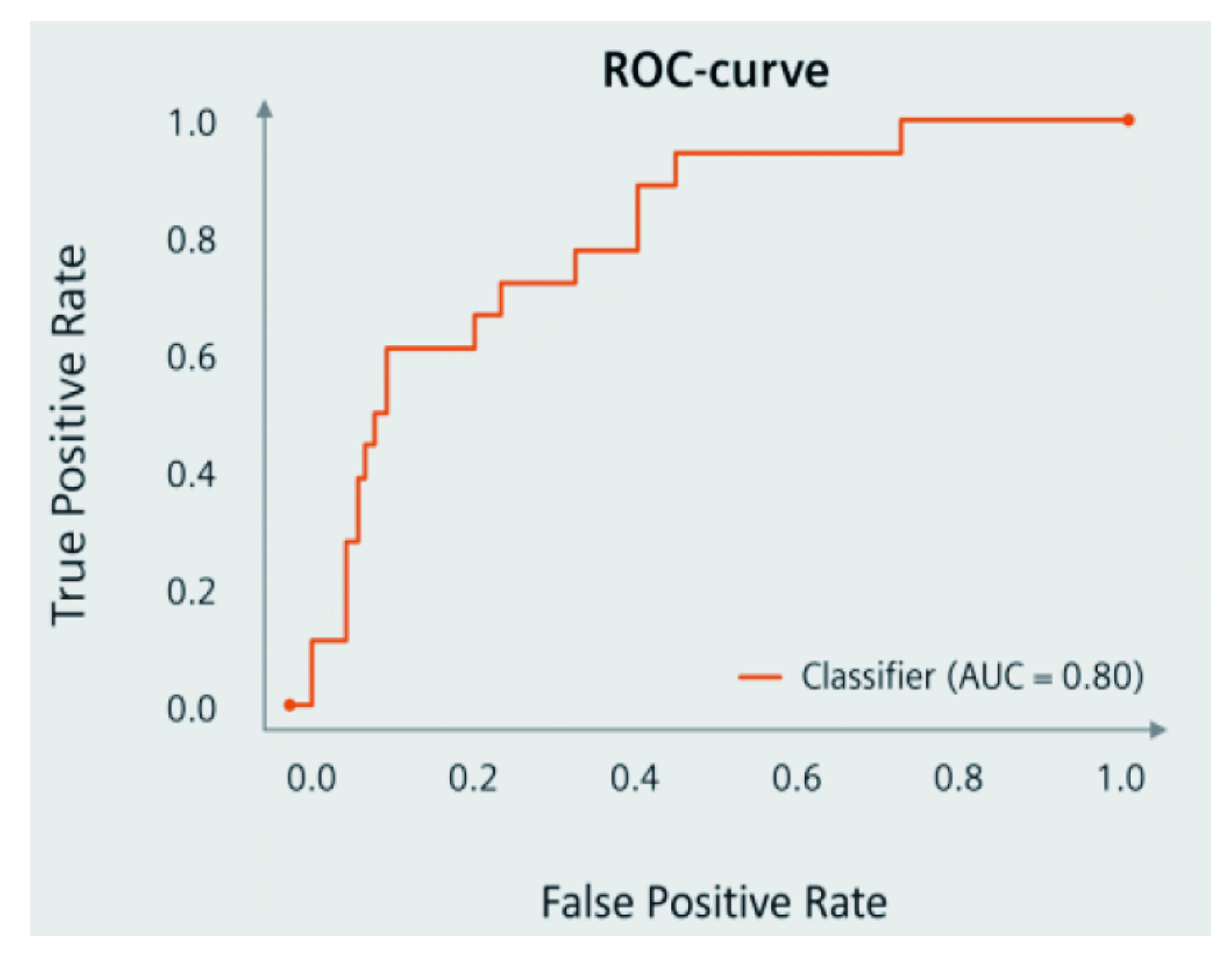
Figure 3. ROC curve on AI-powered CDSS in predicting RA flarese11
Currently, the rate of sustained remission in RA is reported to be around 30%12, indicating room for improvement either by new therapeutics, or optimised treatment strategies. As demonstrated in Labinsky's study, the support for frontline physicians in making the appropriate clinical decision is limited and treatment protocols are inconsistent. Emerging data suggest the potential of AI-powered tools in predicting disease activity in RA. With further enhancement in accuracy, usability, and acceptance, the technology is expected to provide better clinical decision support for physicians, and improved patients' outcomes.
References
1. Lwin et al. Rheumatol Ther 2020; 7: 457. 2. Vodencarevic et al. Arthritis Res Ther 2021; 23: 1–8. 3. Van der Leeuw et al. Arthritis Res Ther 2022; 24: 1–11. 4. Van Mulligen et al. Ann Rheum Dis 2020; 79: 1174. 5. Van Herwaarden et al. BMJ 2015; 350. DOI:10.1136/BMJ.H1389. 6. Chaiamnuay et al. Medicine 2022; 101: E29974. 7. Tanner et al Arthritis. Arthritis Rheumatol 2019; 71: 1494–503. 8. Momtazmanesh et al. Rheumatol Ther 2022; 9: 1249. 9. Chung et al. BioData Min 2021; 14. DOI:10.1186/S13040-021-00284-5. 10. Haschka et al. Ann Rheum Dis 2016; 75: 45–51. 11. Labinsky et al. Diagnostics (Basel) 2023; 13. DOI:10.3390/DIAGNOSTICS13010148. 12. Kalweit et al. PLoS One 2021; 16: e0252289.





