
Atopic dermatitis (AD) is a chronic inflammatory disease characterized by dry skin, eczematous lesions and lichenification1. The disease has a complex aetiology involving genetic, environmental and immunological factors; recent evidence suggests that it is a systemic disorder, more than the interactions between skin barrier defects and immune dysregulation1,2. As one of the most common skin disorder globally, AD affects up to 4.9% of adults2,3. The burden of disease not only taxes on patients’ physical and psychological wellbeing, but also spills over to their family and wider society in terms of healthcare expenditure and productivity loss2,3.
Latest European Guidelines Updates
Disregarding the complementary alternative medicine that should be avoided, the treatment of AD is constituted by three major components: trigger avoidance, daily skin care, and anti-inflammatory therapy1,4. For mild disease, it can be effectively treated by topical emollients and anti-inflammatory agents, while patients with moderate-to-severe disease often attain suboptimal long-term outcomes because of lacking effective systemic treatment5. Of all AD patients, more than 30% of them have reported disease severity as moderate-to-severe3. The situation demonstrated here therefore suggests a significant unmet medical need.
Referring the 2018 consensus-based European guidelines for treatment of AD, treatment recommendations are built on top of basic therapy, including education, skin care practice and allergen avoidance, and consists of three levels; each level corresponds to respective severity with the composite score ‘SCORAD’ and definition for denotation: mild – SCORAD <25 / or transient eczema, moderate – SCORAD 25-50 / or recurrent eczema, and severe – SCORAD>50 / or persistent eczema4. Mild-to-moderate disease involves mainly the use of topical agents and phototherapy, whereas severe disease requires higher level of care, such as systemic immunosuppression and hospitalization4. At the time, there were only a few treatment options with licensed indication for patients with severe
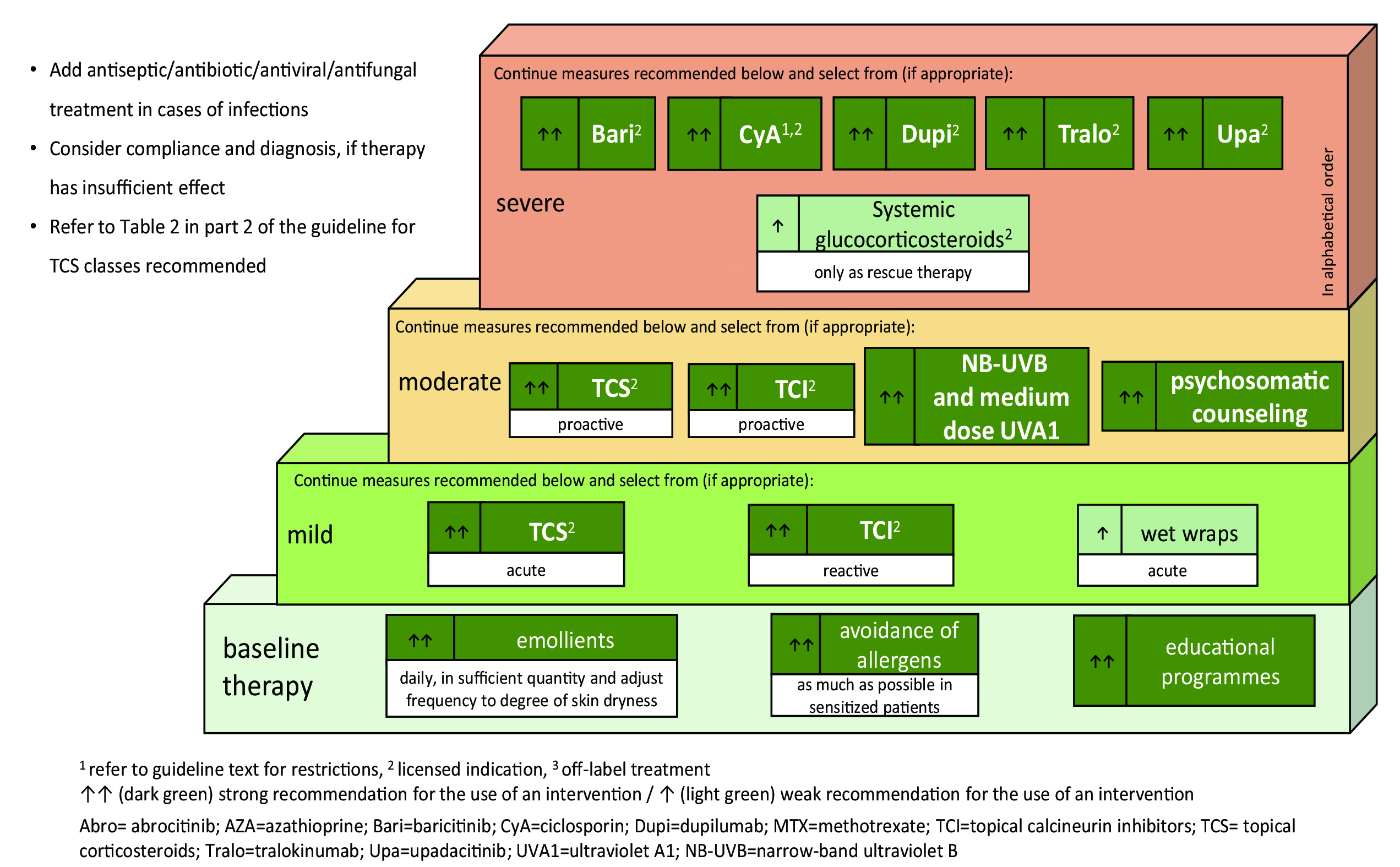
Figure 1. Stepped-care plan for adults with atopic eczema6
disease upon the recommendation, namely cyclosporine A, oral glucocorticosteroids and dupilumab4. In the course of time, treatment choices for severe AD have been broadened in 2022 summer. The European Dermatology Forum released the EuroGuiDerm guideline on AD, updating on systemic therapy6. Three new options, baricitinib, tralokinumab, and upadacitinib, were added to the line of above-mentioned agents6. The stepped-care plan of EuroGuiDerm guideline adopts the overall structure of previously discussed 2018 one, but strength of recommendation has been further integrated into it for finer expression (Figure 1)6. Among the newly recommended options, two of them are the members of Janus kinase (JAK) inhibitor, and the remaining belongs to the class of monoclonal antibody. JAK inhibitor is already on use for treating other immune-mediated diseases before recently approved for AD treatment as a new drug class7. This update on guideline may bring about another change in treatment paradigm.
JAK Inhibitors in a Nutshell
The new joiner – JAK inhibitor includes both topical and systemic oral therapies8, however the following will solely focus on systemic therapy. In brief, the drug works by inhibiting the JAK receptors and downstream cytokine pathways involved in the pathogenesis of AD8. There are four JAK proteins and seven STAT proteins for the targeted JAK-STAT pathway. JAK1 and JAK3, amidst the eleven proteins, are considered responsible for mediating the signalling of IL-4 cytokine which plays an important role in AD cascade7. Especially, the inhibition of JAK1 signalling pathways helps to control chronic itch or pruritus7,8.
Concerning efficacy, JAK inhibitors demonstrated favourable results in following outcome responses, EASI, IGA, and pruritus-NRS9--11. Patients treated with JAK inhibitors presented a significant decrease in EASI score compared with the placebo/vehicle group10, meanwhile the proportion of patients achieving EASI-75 response was higher in JAK inhibitors group than those with placebo9. Similar trend was observed for IGA response9,10. The largest improvement in IGA response occurred at week 411. In terms of pruritus, JAK inhibitors-treated patients achieved 4-point reduction in Peak Pruritus-NRS score (10-point rating scale, with 0 being “no itch” and 10 being “worst itch imaginable”) as early as 1 to 2 weeks11. Furthermore, some studies suggested slightly numerical superiority of oral JAK inhibitors over dupilumab in efficacy during head-to-head comparison7. In short, they have proven their ability in AD symptom resolution for short and medium-term alongside with rapid onset of action7, but still need further data to address concern over long-term efficacy7,9-11.
Regarding the safety profile, most treatment emergent adverse events were mild and transient in nature, and amenable to symptomatic treatment, suggesting reassurance11. The most common adverse events (AEs) reported were headache, nausea, nasopharyngitis and upper respiratory tract infection7. Nevertheless, increased blood creatine phosphokinase was observed in the trials with all oral JAK inhibitors (abrocitinib, baricitinib, and upadacitinib); as yet the association with clinical manifestation of myopathy or rhabdomyolysis has not been found in previous stuides11. Studies on treatment of a fair few JAK inhibitors for rheumatoid arthritis (RA) disclose the increased risks of thromboembolic events and major cardiovascular problems in patients8,9 ,11. But severe AEs occur less in younger and relatively healthier individuals with AD than elderly individuals or those predisposed to autoimmune conditions like psoriasis or RA8,11. Anyway, it is important to heed the involvement of the targeted JAK/STAT signalling pathway in several physiological regulatory processes that may potentially increases the risk of side effects7. Additional evidence will be necessary for detailing a more comprehensive profile on both efficacy and safety through head-to-head comparison and real-world trials7.
Future Treatment Paradigm and Aspiration
The current treatment paradigm can somehow be reflected by the respective market share of the administration route, with topical agents in a leading position. The market size of AD treatment is expected to double in 5 years’ time, and the greatest growth will come from oral agents12. This expeditious growth rate means that oral agents are stepping into a bigger role in AD treatment and could also be perceived as a proxy of expectations from patients for simple but potent treatment. Additionally, the growth might stimulate investment in research of the drug class, which would in turn generate data advising more informed clinical decisions for better patient outcomes upon switching therapy. So, whether JAK inhibitors can earn them an advantageous position depends profoundly on the study results in the coming years.
EASI = Eczema Area and Severity Index. IGA = Investigator Global Assessment. JAK-STAT = Janus kinase-signal transducer and activator of transcription. Pruritus-NRS = Pruritus-Numerical Rating Scale. SCORAD = SCORing Atopic Dermatitis.
References
1. Kolb L & Ferrer-Bruker SJ. Atopic Dermatitis. StatPearls. Available at https://www.ncbi.nlm.nih.gov/books/NBK448071/. Accessed Aug 23, 2022. 2. Xue L, et al. Front Cell Infect Microbiol 2022; 12: 861053. 3. Barbarot S, et al. Allergy 2018; 73(6): 1284-1293. 4. Wollenberg A, et al. J Eur Acad Dermatol Venereol 2018; 32(5): 657-682. 5. Guttman-Yassky E, et al. Expert Opin Biol Ther 2013; 13(4):549-561. 6. Wollenberg A, et al. J Eur Acad Dermatol Venereol 2022; 36(9): 1409-1431. 7. Nogueira M & Torres T. Dermatol Pract Concept 2021; 11(4): e2021145. 8. Singh R, et al. Immunotargets Ther 2020; 9: 255-272. 9. Tsai HR, et al. J Pers Med 2021; 11(4): 279. 10. Li C, et al. Dermatology 2022; 238: 725-735. 11. Le M, et al. Front Med 2021; 8: 682547. 12. Market Data Forcast. Atopic Dermatitis Market. Available at https://www.marketdataforecast.com/market-reports/atopic-dermatitis-treatment-market/. Accessed Aug 26, 2022.





