
Combating Clostridioides Difficile Infection by Microbiota-based Therapy
Clostridioides difficile infection (CDI) is the leading cause of antibiotic-associated diarrhoea and is associated with significant morbidity and mortality. In Hong Kong, the estimated crude incidence rate of CDI is alarming that, among adults aged ≥65 years, 133 to 207 cases per 100,000 population have been reported and an annual increase of 26% in CDI was noted from 2006 to 20141. While domination and colonisation of Clostridioides difficile in the large intestine upon disruption of the balance of gut microbiota is reported as the first step of CDI2, intestinal microbiota restoration by microbiota-based therapy (MBT) has been applied as a treatment for disorders caused by intestinal dysbiosis1-3. Particularly, recent investigations reported that RBX2660, a MBT of suspension of healthy donor microbiota, yielded promising treatment outcomes against CDI.
Increased Risk of CDI during Antibiotic Treatment
Antibiotic exposure, older age (≥65 years), and hospitalisation are the known factors for CDI development. Particularly, former study by Hensgens et al (2012) on hospitalised patients reported that, during antibiotic therapy and in the first month after cessation of the therapy, the patients had a 7 to 10-fold increased risk for CDI (odds ratio [OR]: 6.7-10.4). However, the risk declined in the period between 1 and 3 months after cessation of antibiotics (OR: 2.7)4. Under unfavourable conditions, such as lack of nutrients and other stress factors, Clostridioides difficile can survive by producing dormant spores, which are resistant to heat, acid and antibiotics, within hospital environment or in the host5. The production of spores thus allows Clostridioides difficile to persist in the host during antibiotic treatment. Hence, suppression of gut microbes with antibiotics would possibly alter indigenous gut microbiome and subsequently produce a favourable environment for inducing CDI. Moreover, the altered indigenous gut microbiome also influences the bile acid composition in colon, which promotes the growth of Clostridioides difficile6 leading to increased risk of recurrent CDI, an episode of CDI occurring within 8 weeks of a previous episode7.
Clinical Manifestations of CDI
The clinical outcomes of CDI can be highly heterogeneous, ranging from asymptomatic carrier state, mild or moderate diarrhoea, to life-threatening fulminant colitis. The incubation period of CDI is very individual-dependent as well, though it is typically 2 to 3 days. Watery diarrhoea is common among patients with CDI. Nonetheless, symptoms including abdominal pain, fever, nausea and vomiting, weakness, and loss of appetite are frequently reported. Of importance, severe cases of CDI are life-threatening, with the occurrence of significant dehydration, hypoalbuminemia with peripheral oedema, and subsequent circulatory shock. Additionally, intestinal paralysis, kidney failure, and even death are possible in severe CDI2. Sidler et al (2014) reported that mortality associated with CDI complications varied between 19.7% and 36.7% in patients in intensive care units (ICU)8.
Importance of the Balance in Gut Microbiota
As discussed, the interference on the composition of gut microbiota by antibiotics would lead to overgrowth of Clostridioides difficile and toxin production, and eventually causing CDI6. This highlights the importance of maintaining the balance in gut microbiota. In fact, the gut microbiome is a complex ecosystem consisting of over 1,000 bacterial species reaching its highest concentration in the colon. These commensal bacteria are essential for the host metabolism, nutrition function, maturation of the immune system and protection against pathogens9. Nonetheless, many factors would disrupt the balance of gut microbiota, a condition known as intestinal dysbiosis. Apart from the use of antibiotics, diet, geography and development of gastrointestinal disease can influence the composition of the gut microbiota as well9.
Clinical Efficacy of Microbiota-based Therapy in Countering CDI
Upon the development of CDI-associated symptoms, timely treatment for controlling symptoms and restoring the normal composition of gut microbiota is essential. In local clinical practice, the first-line treatment for non-fulminant Clostridioides difficile-associated diarrhoea includes the cessation of unnecessary medications and treatment with vancomycin or fidaxomicin. For more severe cases, a combination of vancomycin and intravenous metronidazole with early consideration of surgery may be required1. On the other hand, MBT has be reported to be effective in restoring the balance of gut microbiota, and controlling disorders caused by intestinal dysbiosis including CDI10. While the wide spectrum of physiological roles played by gut microbiota has been discovered, the potential therapeutic applications of MBT in various diseases, such as inflammatory bowel disease (IBD), functional gastrointestinal disorders, and metabolic syndrome, have been investigated and reported.
In the randomised control trial by Dubberke et al (2018), the efficacy of RBX2660, a MBT prepared from suspensions of healthy donor microbiota, in restoring the diversity of gut microbiota and preventing recurrent CDI was investigated. In the trial, 133 patients with a history of 2 or more recurrent CDI from 21 centres in the United States and Canada were randomly assigned to receive 2 doses of RBX2660, 2 doses of placebo, or 1 dose of RBX2660 followed by 1 dose of placebo. Administration of the first dose commenced 24-48 hours following completion of CDI treatment antibiotics, with the second dose administered 7 ¡Ó 2 days thereafter. Treatment success was defined as absence of CDI recurrence at 8 weeks post administration of assigned study treatment11.
The results showed that, at 8 weeks after the second dose of assigned study treatment, 1 dose of RBX2660 (n=42, 67%) yielded superior efficacy in preventing recurrent CDI as compared to placebo (n=44, 45%, p=0.049), whereas the efficacy of 2 doses of RBX2660 (n=41, 61%) was higher than that of placebo, but the difference was not statistically significant (p=0.152, Figure 1). Further, the results reflected no significant difference in the occurrence of adverse events among the 3 groups, showing that RBX2660 is safe and well-tolerated11.
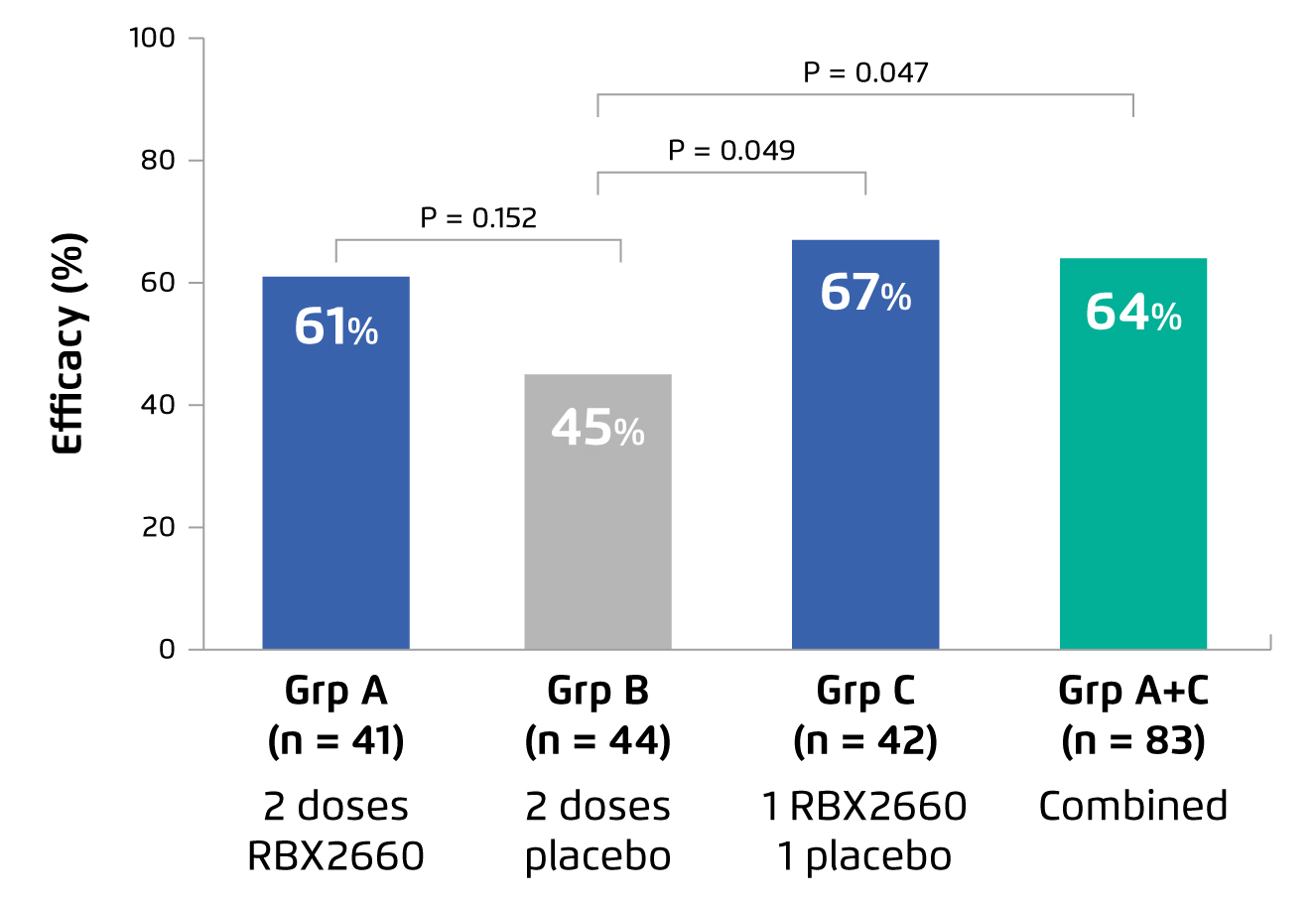
Figure 1.
Efficacy of RBX2660 or placebo in preventing recurrent CDI11
While the inclusion criteria in prospective clinical trials are often narrowly defined, a recent retrospective analysis of real-world setting by Feuerstadt et al (2021) demonstrated that the treatment success rate, defined as absence of CDI recurrence at 8 weeks post administration of treatment, achieved by RBX2660 was 82.8%, with no observable difference between patients who received 1 dose (83.3%) versus 2 doses (82.5%). Among patients who responded to RBX2660 treatment after the first dose, 88.7% demonstrated a sustained clinical response to 6 months. Besides, the safety outcomes of RBX2660 in this analysis were comparable to those reported in former clinical trials12.
Remarks
There are emerging quality evidence suggesting the efficacy and safety of MBT in controlling CDI. In view of the lacking of effective alternatives for difficult-to-treat cases of CDI, the clinical significance of MBT is expected to increase. Further investigations on treatment kinetics, dosing and mechanisms of action would help optimising the clinical use of MBT.
References
1. Lui et al. Hong Kong Med J 2019; 25: 178-82. 2. Czepiel et al. Eur J Clin Microbiol Infect Dis 2019; 38: 1211. 3. Smits et al. Nat Rev Dis Prim 2016; 2: 16020. 4. Hensgens et al. J Antimicrob Chemother 2012; 67: 742-8. 5. Setlow. J Appl Microbiol 2006; 101: 514-25. 6. Britton et al. Gastroenterology 2014; 146: 1547. 7. Song et al. Gut Liver 2019; 13: 16. 8. Sidler et al. Swiss Med Wkly 2014 39 2014; 144. DOI:10.4414/SMW.2014.14009. 9. Schäffler et al. Front Microbiol 2018; 0: 646. 10. Baunwall et al. EClinicalMedicine 2020; 29-30. DOI:10.1016/J.ECLINM.2020.100642. 11. Dubberke et al. Clin Infect Dis 2018; 67: 1198-204. 12. Feuerstadt et al. ACG 2021: P2217.





