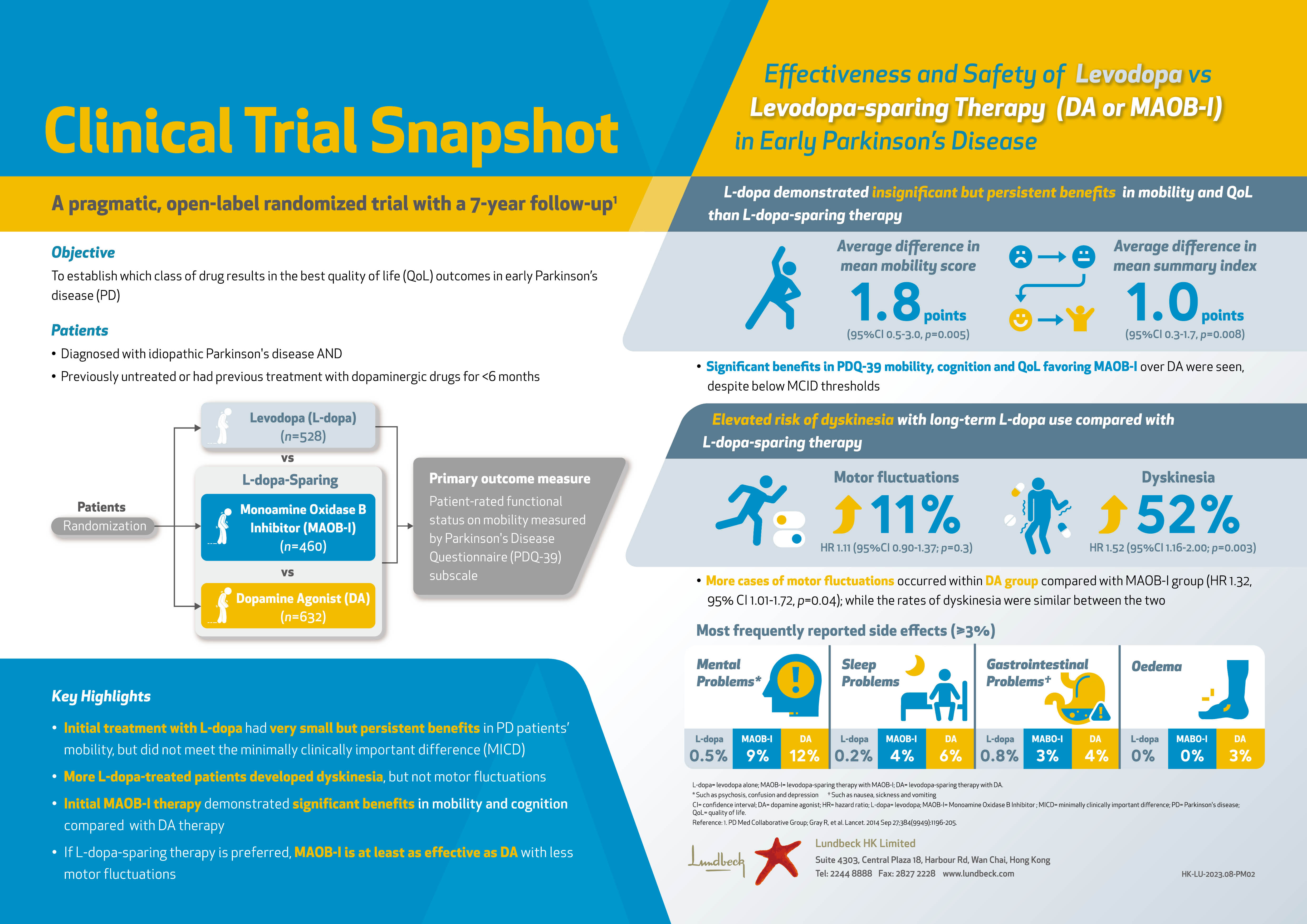
Exkivity®
(mobocertinib)1-3 Takeda
References:
1. EXKIVITY 40 mg hard capsules. EMC. Published 2023. Accessed May12, 2023. https://www.medicines.org.uk/emc/product/13468/smpc#gref
2. EXKIVITY (mobocertinib) capsules. FDA. Published 2021. Accessed May15, 2023. chrome- extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215310s000lbl.pdf
3. FDA grants accelerated approval to mobocertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations. FDA. Published 2021. Accessed May15, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20