Immune Checkpoint Inhibitor Therapy in Patients with Autoimmune Disorders: A Brief Review
The clinical development of immune checkpoint inhibitors (ICIs), including those targeting the programmed cell death 1 and its ligand 1 (PD-1/PD-L1), or cytotoxic T-lymphocyte antigen 4 (CTLA-4), have led to a paradigm shift in cancer treatment. By disrupting the PD-1/PD-L1 and CTLA-4 pathways, T-cells can be relieved from the tumour-directed inhibition1. However, ICIs were found to increase the risk of systemic immune-related adverse events (irAEs), especially in patients with autoimmune disorders (AIDs). To minimise potential immunotoxicity, several clinical trials of ICIs excluded this subgroup of patients2-4. For further understanding, this article briefly summarises the studies of ICIs in patients with AIDs and discusses the safety profile of ICIs use in this population.
Immune Checkpoint and Tumour Escape
Naturally, the PD-1/PD-L1 and CTLA-4 pathways give negative feedback and downregulate immune responses for preventing immunopathology1,5. The mechanisms could be utilised by cancer cells to evade and suppress the immune system, which favours their survival and proliferation. In the tumour microenvironment, PD-L1 is expressed by cancer cells and interacts with PD-1 receptors on the surface of T-cells, and consequently, downregulates T-cell activation and reduces cytokines production. Moreover, the expression of PD-1 and CTLA-4 are increased in exhausted tumour-infiltrated T-cells. CTLA-4, a homologue of CD28, binds CD80/86 on antigen-presenting cells (APCs), thereby downregulating T-cell activation.
With ICIs, this inhibitory effect is reverted and the tumour-directed immune responses could be restored (Figure 1)1,5. This was proven by several clinical trials, supported by the improvement of survival rate in multiple types of cancer, such as non-small cell lung cancer (NSCLC), urothelial cancer, and melanoma (Table 1)2,6,7. Although ICIs provide encouraging clinical outcomes, their efficacy and safety profiles remain unclear in patients with pre-existing AID.
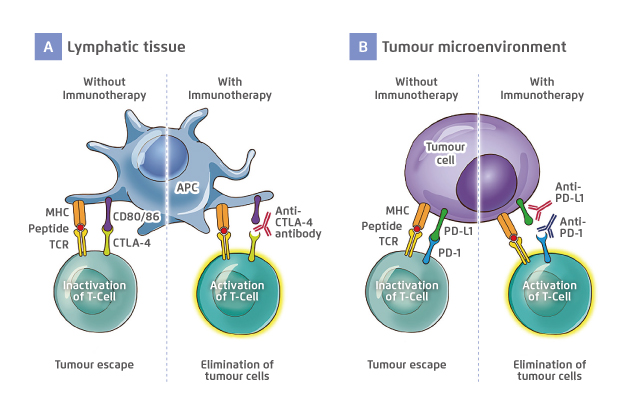
Figure 1. The role of (A) CTLA-4 and (B) PD-1/PD-L1 and the mechanisms of corresponding ICI
Figure adapted from Soularue E, et al5
APC: antigen-presenting cell; CD: cluster of differentiation; CTLA-4: cytotoxic T-lymphocyte antigen 4; ICI: immune checkpoint inhibitor; MHC: major histocompatibility complex; PD-1: programmed cell death 1; PD-L1: programmed cell death ligand 1; TCR: T-cell receptor
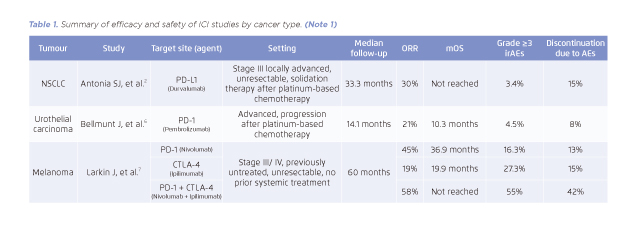
Table 1. Summary of efficacy and safety of ICI studies by cancer type. (Note 1)
Efficacy of ICI Therapy in Patients with AID
The associations between pre-existing AIDs and overall survival (OS) as well as objective response rate (ORR) upon PD-L1 therapy were evaluated in a prospective study involving 45 patients with AID on PD-L1 inhibitor8. The most frequent pre-existing AIDs among the patients were vitiligo (n=17), psoriasis (n=12), thyroiditis (n=7), Sjogren syndrome (n=4), and rheumatoid arthritis (n=2). The patients had received a median of 1 previous treatment for their AIDs. At the last follow-up, 9% and 27% of patients achieved complete response (CR) and partial response (PR) respectively, while 18 deaths due to cancer progression were recorded. The OS achieved by ICI therapy in patients with AIDs was comparable to those without AIDs (p=0.38). Nonetheless, a greater ORR was observed in patients with AIDs (38%, 9% with CR and 29% with PR) as compared to AID-free patients (28%, 7% with CR and 21% with PR)8.
Safety Profile in Patients with AID
In a nationwide cohort study from France9, the occurrence of flare and irAEs was reviewed in 112 patients with pre-existing AIDs after receiving ICIs. At baseline, the most frequent AIDs were psoriasis (n=13), rheumatoid arthritis (n=20), and inflammatory bowel disease (n=14). In addition, 22% of patients (n=24) were receiving immunosuppressants at the initiation of ICI therapy. With a median follow-up of 8 months, the flare of AIDs or irAEs occurred in more than 40% of patients, whereas most of them were manageable (Table 2)9.
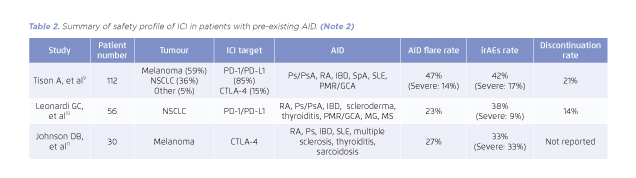
Table 2. Summary of safety profile of ICI in patients with pre-existing AID. (Note 2)
The rate of severe irAEs (grade 3 or above) was lower in patients treated with anti-PD-1/PD-L1 than those treated with anti-CTLA-4 (9% vs. 33%) but the rate of flare of pre-existing AIDs remained comparable (23% vs. 27%)10,11. However, owing to the small numbers of AID patients treated with anti-CTLA-4, further studies should be conducted to evaluate the risk of immunotoxicity in this population.
Recommendations on ICI Therapy for AID Patients
Based on the limited safety data in the setting of pre-existing AID, National Comprehensive Cancer Network (NCCN) has published recommendations in regard to patients who may be suitable for ICI therapy. The recommendations suggest that ICIs may be considered in patients with low or without immunosuppressant or good control of AID. However, it should be avoided in patients who require high doses of immunosuppressant or have poor control of AID1,12. Patients with neurologic AIDs, such as myasthenia gravis, or life-threatening AIDs may not be considered good candidates for ICI treatment. Although pre-existing AID is not contraindicated to ICI therapy, ICIs should be used with caution owing to the development of immunotoxicity1,12.
(Note 1)
Does not include all ICI studies and for information purpose only. For more details, please refer to the cited articles.
AE: adverse event; CTLA-4: cytotoxic T-lymphocyte antigen 4; ICI: immune checkpoint inhibitor; mPFS: median progression-free survival; mOS: median overall survival; NSCLC: non-small-cell lung cancer; ORR: overall response rate; PD-1: programmed cell death 1; PD-L1: programmed cell death ligand 1.
(Note 2)
Does not include all studies and for information purpose only. All studies listed were retrospective. For more details, please refer to the cited articles.
AID: autoimmune disorders; CTLA-4: cytotoxic T-lymphocyte antigen 4; GCA: giant cell artheritis; IBD: inflammatory bowel disease; ICI: immune checkpoint inhibitor; irAEs: immunotherapy-related adverse events; MG: myasthenia gravis; MS: multiple sclerosis; NSCLS: non-small-cell lung cancer; PD-1: programmed cell death 1; PD-L1: programmed cell death ligand 1; PMR: polymyalgia rheumatic; Ps: psoriasis; PsA: psoriatic arthritis RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; SpA: spondylosing arthropathy.
References
1. Kennedy LC, et al. J Natl Compr Canc Netw. 2019; 17: 750-757. 2. Antonia SJ, et al. N Engl J Med. 2017; 377: 1919-1929. 3. Borghaei H, et al. N Engl J Med. 2015; 373: 1627-1639. (NSCLC) 4. Motzer RJ, et al. N Engl J Med. 2018; 378: 1277-1290. 5. Soularue E, et al. Gut. 2018; 67: 2056-2067. 6. Bellmunt J, et al. Engl J Med. 2017; 376: 1015-1026. 7. Larkin J, et al. N Engl J Med. 2019; 381: 1535-1546. 8. Danlos F, et al. Eur J Cancer. 2018; 91: 21-29. 9. Tison A, et al. Arthritis Rheumatol. 2019; 71: 2100-2111. 10. Leonardi GC, et al. J Clin Oncol. 2018; 36: 1905-1912. 11. Johnson DB, et al. JAJA Oncol. 2016; 2: 234-240. 12. Thompson JA, et al. J Natl Compr Canc Netw. 2019; 17: 255-289.