
The Therapeutic Efficacies of Deep Brain Stimulation on Treatment-resistant Depression
Major depressive disorder (MDD) is one of the most common psychiatric disorders in specialist and general practice. MDD can present at any age across the life span. Essentially, depressive symptoms are debilitating and, at its worst, can be life threatening1. In Hong Kong, according to the Mental Morbidity Survey which involved 5,700 Chinese adults aged 16-75, the weighted 1-week prevalence of depressive episode was 2.9%. Moreover, about 6.9% of respondents had mixed anxiety and depressive disorder2. Although various antidepressant treatments have been developed, at least 30% of patients with MDD demonstrate treatment resistance to antidepressants3. Treatment-resistant depression (TRD) is commonly defined as MDD which does not respond to a minimum of 2 prior treatments with confirmation of prior adequate dose and duration4. In order to manage TRD, various treatment approaches have emerged. Particularly, deep brain stimulation (DBS) has been demonstrated to be a safe and effective therapy for TRD.
Non-pharmacological Therapeutic Options for TRD
Psychotherapeutic approaches may be applied in combination with somatic or pharmacological treatments. Besides, these approaches can be undertaken on their own once several other interventions have been attempted1. Recent meta-analysis suggested that inclusion of add-on of psychotherapy to usual treatment would improve treatment outcome for patients with TRD as compared to those only receiving the usual treatment5. Nonetheless, further evidence on the effectiveness of different types of psychotherapies in TRD is needed. Moreover, there is no solid evidence showing the benefit of switching to psychotherapy from antidepressant medication among patients with TRD6.
Electroconvulsive therapy (ECT) was shown to restore depressive effects triggered by stress in animal models7 and in randomised controlled trials (RCT)8. It is suggested that ECT induced antidepressant properties by decreasing neural metabolic activity, which was due to the enhanced gamma-aminobutyric acid (GABA) transmission9. However, former report demonstrated that the efficacy and side effects of ECT were significantly associated with electrode placement and dosage8. As compared to ECT, repetitive transcranial magnetic stimulation (TMS) is a relatively new form of brain stimulation treating TRD. The treatment utilises focused pulses of an electromagnetic coil repetitively discharged over the scalp to stimulate cortical neurons and alter neural excitability without a seizure1. O’Reardon et al (2007) demonstrated the efficacy of TMS on acute major depression in a RCT involving 201 patients with MDD. The results demonstrated that active TMS was significantly superior to sham TMS in treating MDD, as defined by the Montgomery-Asberg Depression Rating Scale (MADRS), with minimal side effects10.
Therapeutic Efficacies of DBS in TRD
DBS is a targeted therapeutic alternative for TRD which involves bilateral placement of electrodes at specific neuroanatomical sites to deliver continuous stimulation from a subcutaneously implanted pulse generator11. Currently, there are several regions that have been investigated as targets in TRD, namely nucleus accumbens (NAcc), ventral capsule and striatum (VC/VS), and the subgenual cingulate cortex (SCC)1. The selection of these structures has been supported mainly by neuroimaging and lesional studies. Preclinical animal studies also provided insights on the selection of targets for DBS. For instance, it has been suggested that SCC is metabolically overactive in TRD.
Hence, Mayberg et al (2005) investigated the modulation of SCC by chronic DBS in SCC in order to control TRD. The placement of implanted electrodes in SCC for DBS is illustrated in Figure 1. Upon 6 months of DBS, striking and sustained remission of depression was achieved in 4 of the 6 patients. The results of PET scanning further suggested that the antidepressant effects were associated with a marked reduction in local cerebral blood flow (CBF) and changes in downstream limbic and cortical sites (Figure 2)11.
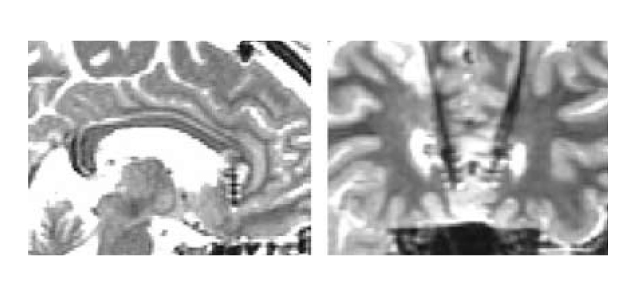
Figure 1. DBS electrode placement in SCC,(A) sagittal and (B) coronal views11
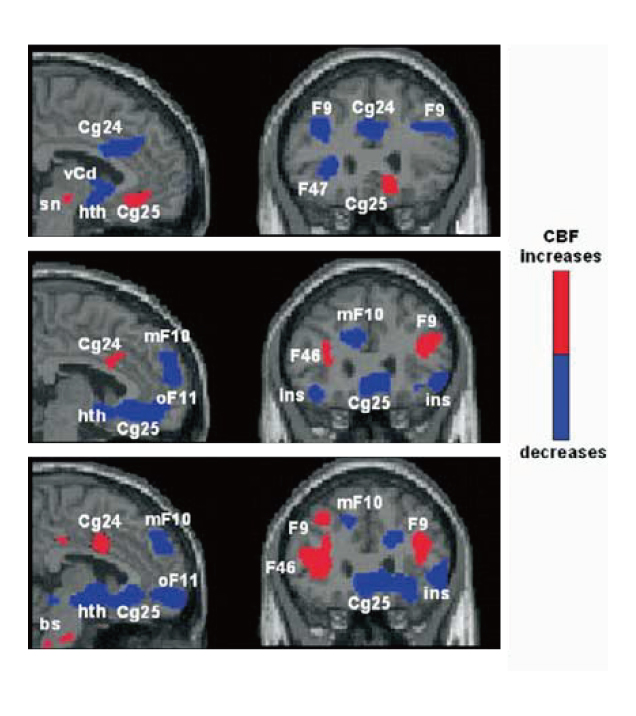
Figure 2. PET scans showing changes in CBF changes upon DBS11
DBS is increasingly being used for treating TRD. However, probably due to its invasive nature, the number of blinded, randomised controlled trials evaluating the therapeutic efficacy of DBS in TRD is limited and the sample sizes of previous trials are small. Moreover, the studies report variable response rates over several months that appear to improve over time, ranging from 29% to 75%11-13. Nonetheless, a recent meta-analysis which reviewed 9 studies, all but 2 were double-blinded RCTs, demonstrated that DBS treatment would generally reduce depressive symptoms. In the pooled data of 190 patients, up to 16 weeks of follow-up, DBS yielded a significantly higher response (odds ratio: 5.50, 95% confidence interval [CI]: 2.79-10.85, p<0.0001) and reductions in mean depression score (standardised mean differences (SMD): -0.42, 95% CI: -0.72- -0.12, p=0.006) as compared to sham control (Figure 3)14. Thus, former trial data demonstrated the therapeutic potential of DBS in controlling depressive symptoms among patients with TRD.
Challenges in TRD Management with DBS
Previous pre-clinical and clinical data revealed the potential therapeutic benefits of DBS in TRD management. However, there are still many obstacles needed to overcome in order to enhance the efficacy as well as to improve the safety profile of DBS treatment. For instance, as an invasive treatment, incidence of hardware infections had been reported and some of the cases could not be solved by replacement of implanted hardware11. Besides, the need of battery replacement surgeries is also a concern. In order to overcome this major drawback, recent attempt on developing non-invasive and focused brain stimulation based on TMS technology has been reported15.
Besides invasiveness, identifying the optimal target brain structures and stimulation parameters for DBS in TRD is another core problem. Summarising the data on stimulus parameters in observational studies on DBS for intractable depression, increasing amplitude followed by changing the electric contacts or increasing pulse width would optimise efficacy. In particular, high frequency stimulation (>100 Hz) was applied in most cases, whereas different combinations of pulse width and amplitude were used for different brain targets16. This further leads to the consideration on the role of personalised medicine in improving treatment outcomes of DBS for TRD.
MDD is a common mental disorder with substantial health burden, whereas a large proportion of patients receiving traditional therapies fail to achieve and sustain remission. Despite technical difficulties still existing, DBS shows promise for TRD and thus could be a therapeutic alternative. Of course, further action for improving the hardware issue and treatment algorithms would be highly desirable.
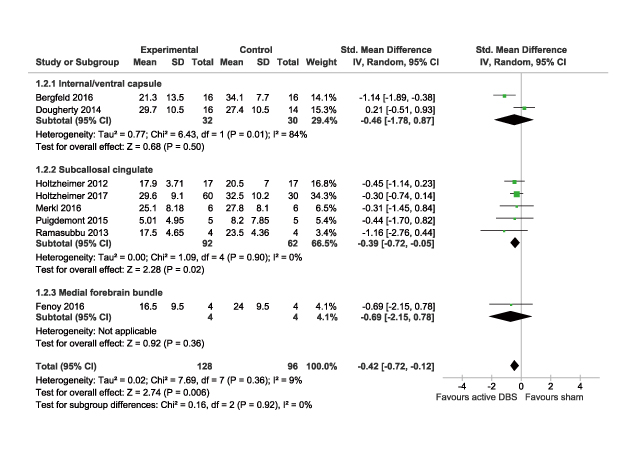
Figure 3. Comparison on mean depression scores in meta-analysis14
References
1. Voineskos et al. Neuropsychiatr Dis Treat. 2020;16:221-234. 2. Lam et al. Soc Psychiatry Psychiatr Epidemiol. 2015;50(9):1379-1388. 3. Rush et al. Am J Psychiatry. 2006;163(11):1905-1917. 4. Gaynes et al. Depress Anxiety. 2020;37(2):134-145. 5. Van Bronswijk et al. Psychol Med. 2019;49(3):366-379. 6. Ijaz et al. Cochrane Database Syst Rev. 2018;2018(5). 7. Katz. Neurosci Biobehav Rev. 1981;5(2):273-277. 8. Merkl et al. Exp Neurol. 2009;219(1):20-26. 9. Sackeim et al. Biol Psychiatry. 1983;18(11):1301-1310. 10. O’Reardon et al. Biol Psychiatry. 2007;62(11):1208-1216. 11. Mayberg et al. Neuron. 2005;45(5):651-660. 12. Lozano et al. J Neurosurg. 2012;116(2):315-322. 13. Raymaekers et al. Transl Psychiatry. 2017;7(10):e1251. 14. Kisely et al. Depress Anxiety. 2018;35(5):468-480. 15. Meng et al. IEEE Trans Magn. 2018;54(5). 16. Ramasubbu et al. Front Psychiatry. 2018;9(JUL):302.





