
Recent Development in Pharmacological Treatment for Cannabis Use Disorder
Cannabis is one of the most commonly used illicit drugs that an estimate of 13.9% of the general population older than 12 reported past-year use in the United States in 2016, whereas the estimate in 2006 was 10.3%1. In Hong Kong, according to the Central Registry of Drug Abuse (CRDA), 8,598 people were reported to have abused drug in 2015 and 4.0% of them abused cannabis2. As many countries have legalised or decriminalised cannabis use, in addition to public health intervention, development in pharmacological treatment for reducing withdrawal and dependence of cannabis is crucial for minimising the burden of cannabis use disorder.
Cannabis and the Endocannabinoid System
Among the over 500 unique chemical components in cannabis, about 100 biologically active compounds are classified as cannabinoids. These compounds are further categorised into 10 classes, 2 of which have dominated research and marketing pursuits: tetrahydrocannabinols (THC) and cannabidiol (CBD) (Figure 1). THC is the primary psychoactive cannabinoid and its effects are largely responsible for the heightened perceptions and anxiety/paranoia reported by the people using cannabis. CBD does not produce psychotomimetic effects, but appears to have anxiolytic, antiepileptic and antipsychotic properties3.
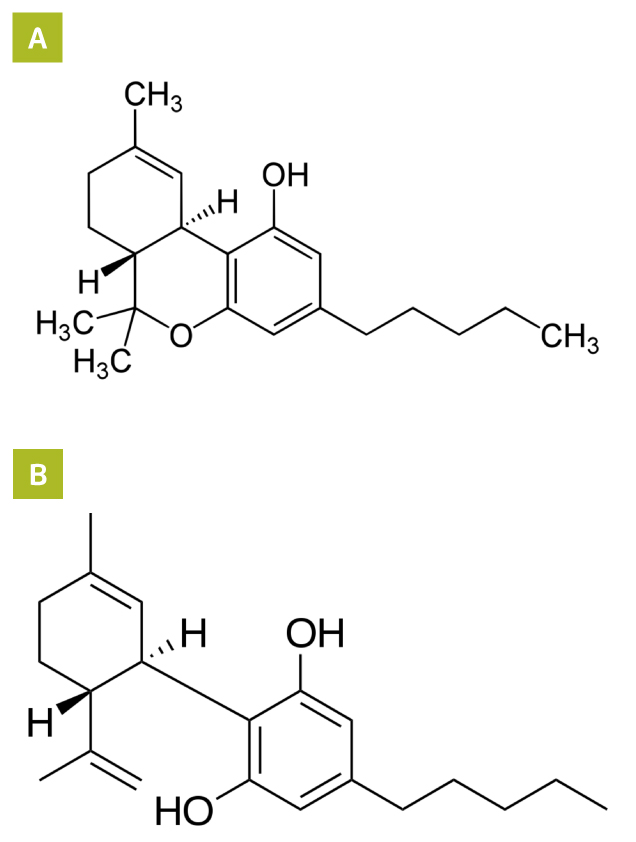
Figure 1. Molecular structures of (A) THC and (B) CBD
The effects of cannabinoids are mediated via the endocannabinoid system (ECS), which consists of endocannabinoid receptors, including CB1R and CB2R, and endogenous ligands, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG). The CB1R is widely distributed in the human brain and its activation is thought to be responsible for psychoactive effects of cannabinoids, whereas the CB2R expresses primarily in peripheral immune cells and the central nervous system. The CB2R is thought to modulate immune function. Besides, AEA is derived from the phospholipid precursor N-arachidonoyl-phosphatidylethanolamine (NAPE) via N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD), while 2-AG derives primarily from the hydrolytic metabolism of 1,2-diacylglycerol (DAG) by sn-1-selective DAG lipases (DAGLα or DAGLβ)4. Both AEA and 2-AG activate endocannabinoid receptors and produce psychoactive effects similar to marijuana5. AEA and 2-AG are synthesised and released on demand. The ligands are rapidly deactivated soon thereafter. AEA is catabolised by fatty acid amide hydrolase (FAAH), whereas 2-AG is catabolised by monoacylglycerol lipase (MAGL) (Figure 2)6.
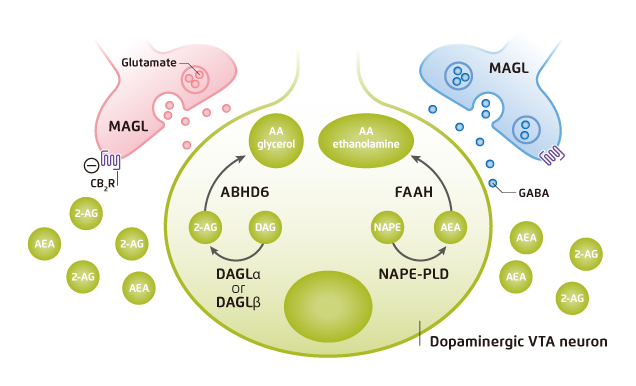
Figure 2. Endocannabinoid biosynthesis, signaling and clearance4 VTA: ventral tegmental area
The Health Burden of Cannabis and Related Compounds
Cannabis produces a wide range of subjective, behavioral, neuropsychological, motor, physiological and neuroendocrine effects in human. Most psychoactive effects of cannabis are, however, attributed to its primary psychoactive constituent, THC3. The most commonly described effect of THC is euphoria. However, cannabinoids would induce perceptual alterations and psychotomimetic effects as well7. Former randomised, double-blind, counterbalanced study showed that exposure to THC would produce a broad range of transient symptoms, behaviors, and cognitive deficits including schizophrenia-like symptoms, altered perception and increased anxiety8. A recent meta-analysis by Marconi et al (2016) demonstrated that higher levels of cannabis use were associated with increased risk for psychosis, with an odds ratio (OR) of 3.90 (95% confidence interval [CI]: 2.84-5.34) as compared to non-users (Figure 3)9.
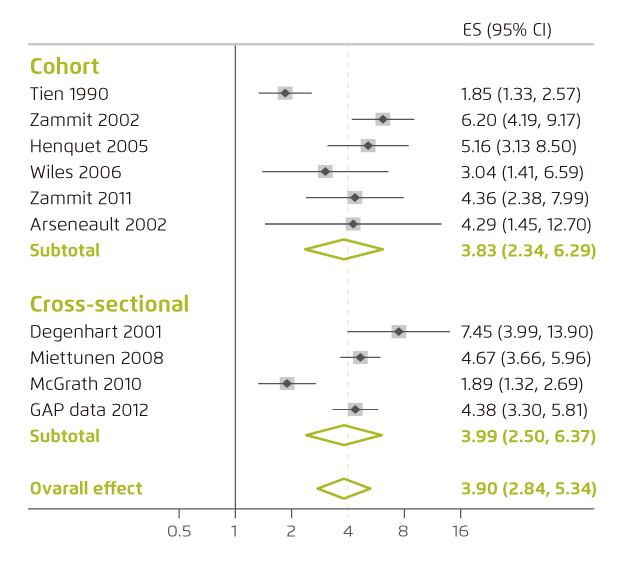
Figure 3. Forest plot of analyses of OR of psychosis in severe cannabis users9. ES: effect size
Recent investigation on the impacts of recent and past cannabis use on neurocognitive functioning involving 158 adult participants demonstrated that recent cannabis users, who consumed cannabis within the past 28 days, exhibited significantly worse cognitive performance than non-users in terms of working memory, information processing speed and executive functioning. Of particular, the results suggested that some of the adverse effects appeared to attenuate with abstinence. However, the neurocognitive functioning of past users was consistently lower than non-users10.
The health burden of cannabis has been intensified by the recent development of synthetic cannabinoids, which are hybrid herbal/chemical products mimicking the effects of cannabis and usually marketed as herbal smoking blend. Their popularity among recreational drug users has grown rapidly because of their easy availability, lower price and, essentially, non-detection by traditional drug tests. Notably, a local case report described the incidence of acute mental disturbance after 4 weeks of daily synthetic cannabinoid abuse in a Chinese recreational drug user11.
Pharmacological Treatment for Cannabis Use Disorder
The global burden of cannabis use is substantial. Of note, 8-12% of regular cannabis users will develop moderate to severe cannabis use disorder (CUD) over time12. However, only a small proportion of individuals with change to CUD receive any type of treatment for their problematic cannabis use. Former evidence suggested that psychosocial treatments for CUD have yielded minimal success, with a high likelihood of relapse13. Hence, effective pharmacotherapy for CUD has been a goal for both researchers and clinicians.
With a better understanding on the roles of ECS in substance use disorder, a variety of pharmacological approaches targeting ECS components for treating CUD has been investigated. For instance, the discovery of rimonabant, a selective CB1R antagonist, is a significant progress in development of medication for treating substance use disorder. However, rimonabant and most of the other CB1R antagonists were withdrawn from clinical trials worldwide in 2008 due to serious adverse effects such as depression and suicidality14.
Potentiating endocannabinoid signalling by inhibiting FAAH is another investigated approach, where the efficacy and safety of PF-04457845, an FAAH inhibitor, in reduction of cannabis withdrawal and cannabis use in men who were daily cannabis users was examined in a recent Phase IIa randomised controlled trial. The results demonstrated that treatment with PF-04457845 was associated with reduced symptoms of cannabis withdrawal and related mood symptoms during the in-patient phase compared to placebo. Additionally, treatment with PF-04457845 was associated with lower self-reported cannabis use at 4 weeks and lower urinary THC-COOH concentrations. Essentially, there were no serious adverse events observed15. Thus, the findings indicated the effectiveness and safety profile of FAAH inhibition in treating CUD.
Say No to Substance Abuse
Substance abuse is a recurrent and chronic condition which, in addition to the substantial socioeconomic burden imposed to the society, would adversely affect physical and mental health as well as quality of life. In the era of cannabis legalisation, safe and effective treatment approaches against CUD are urgently needed. In addition, appropriate public health intervention and social support are essential in order to educate the general public to avoid substance abuse.
References
1. Substance Abuse and Mental Health Services Administration. 2016 National Survey on Drug Use and Health: CBHSQ Data. 2. Narcotics Division Security Bureau. Central Registry of Drug Abuse: Sixty-Fifth Report 2006-2015.; 2015. 3. Bassir et al. Curr Behav Neurosci Reports. 2018;5(4):271-280. 4. Parsons et al. Nat Rev Neurosci. 2015;16(10):579-594. 5. Walentiny et al. Eur J Pharmacol. 2011;656(1-3):63-67. 6. Sloan et al. Neuropharmacology. 2017;124:73-83. 7. Mason et al. Psychol Med. 2009;39(6):951-956. 8. D’Souza et al. Neuropsychopharmacology. 2004;29(8):1558-1572. 9. Marconi et al. Schizophr Bull. 2016;42(5):1262-1269. 10. Thames et al. Addict Behav. 2014;39(5):994-999. 11. Tung et al. East Asian Arch Psychiatry. 2012;22(1):31-33. 12. Perkonigg et al. Addiction. 2008;103(3):439-449. 13. Gates et al. Cochrane Database Syst Rev. 2016;2016(5):CD005336. 14. Galaj et al. CNS Drugs. 2019;33(10):1001-1030. 15. D’Souza et al. The Lancet Psychiatry. 2019;6(1):35-45.





