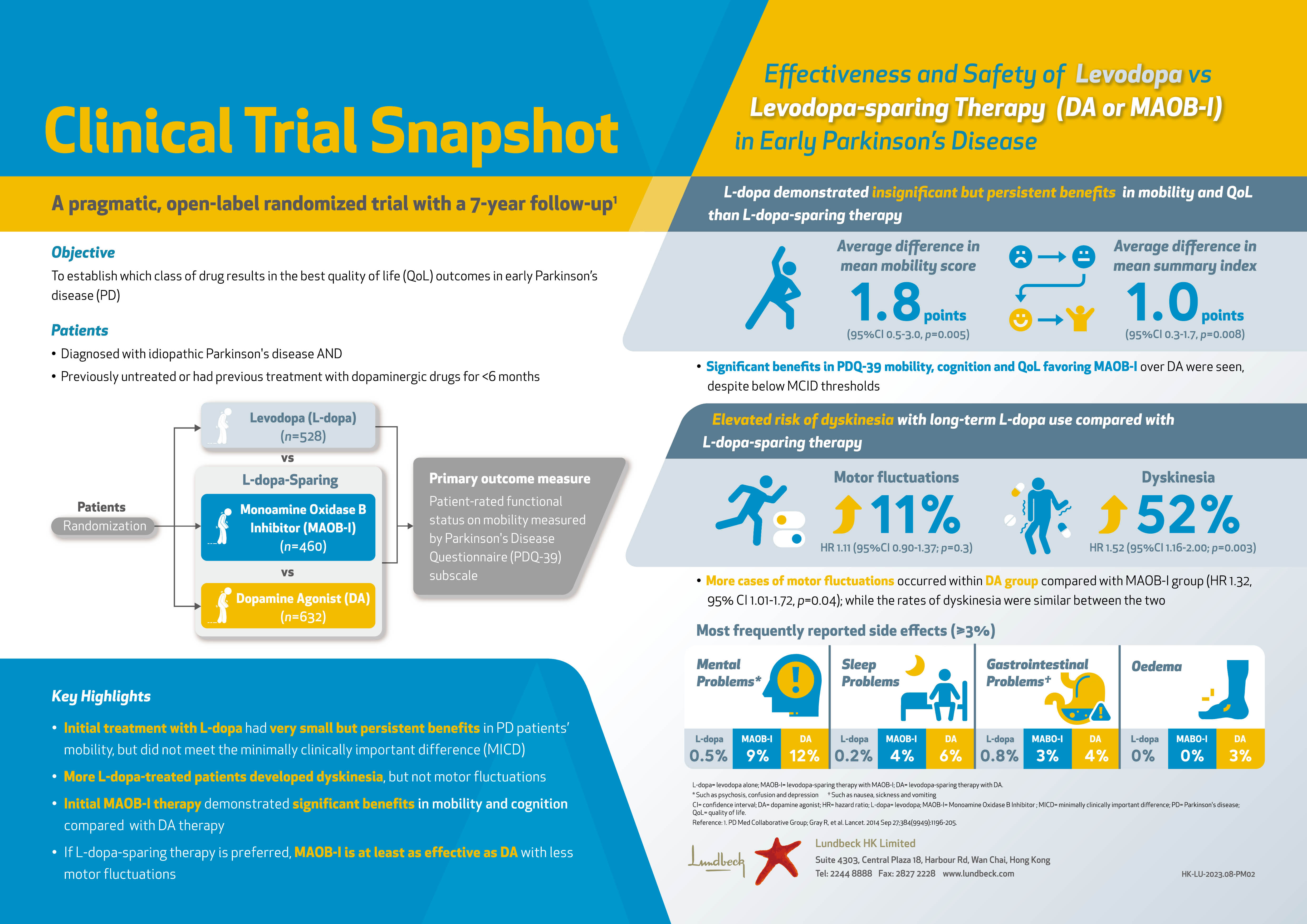
The Applications of Oncolytic Virus in Cancer Therapy
The observation of cancer remission after viral infection was reported in the early 1900s, which led to the hypothesis of treatment against cancer with virotherapy. In fact, there were clinical trials performed between 1950 and 19701. Nonetheless, due to the severe side effects and treatment-related deaths observed, the use of viruses for cancer treatment was considered unsafe. With better understanding on the biology of viruses and cancer cells as well as the technical advancement in viral genome manipulation, the research on virotherapy against cancer revived in 1990s. While T-VEC, a herpes simplex virus type 1 (HSV-1) based oncolytic virus (OV), has been approved by the FDA for melanoma treatment in 20152, the therapeutic potential of OVs drive further development of virotherapy for cancer treatment.
The Programmed Weapons to Fight Cancer: An Overview
Oncolytic virotherapy is the use of a replication-competent virus for the treatment of cancer. Oncolytic viruses (OVs) can selectively infect, replicate within and lyse tumour cells. Newly released OV particles can further infect into uninfected neighbouring tumour cells leading to subsequent waves of oncolytic tumour cell death and induction of a profound systemic anti-tumoural immune response3. Moreover, OVs can kill cancer stem cells and replicate in hypoxic environments as well as in drug-resistant cells4. In addition to direct oncolysis, OVs can act as a cancer vaccine by inducing immunogenic cell death. By releasing tumour-associated antigens (TAAs), cellular damage-associated molecular patterns (DAMPs) and/or pathogen-associated molecular patterns (PAMPs), an anti-tumour immune response is triggered5. Essentially, while OVs preferentially target and kill cancer cells, they have a minimal to no detrimental effect on normal and transformed cells (Figure 1)6.
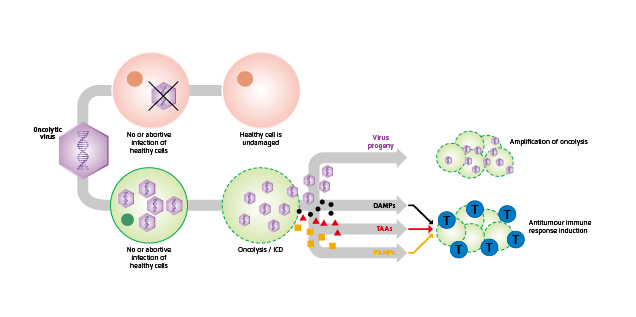
Figure 1. Dual-mode of action of OVs6
The Goals of OV Engineering
Although there are more than 3,000 species of viruses currently identified, not all are suitable as oncolytic agents. The desirable OVs have to be specific for targeted cancer, potent in killing infected cells and triggering anti-tumour immunity. Importantly, the OV has to be safe to avoid adverse reactions and pathogenic reversion7.
The tumour selectivity of OVs can be either natural or obtained by viral genome manipulation. For genetically modified OVs, the viral genome is engineered so that the OVs become more specific towards cancer cells as opposed to their normal counterparts. The most common strategies include expression of modified receptors for cellular entry, restriction of essential viral proteins by using cancer-specific promoters, and deletion of viral proteins that prevent apoptosis in healthy cells8. For instance, recombinant adenoviruses have been modified so that its capsid could incorporate a specific arginine–glycine–aspartic acid (RGD) protein motif (Figure 2). The modification resulted in the ability of the virus to utilise an alternative receptor during the cell entry process and thus leading to an increase in gene transfer to ovarian cancer cells with a magnitude of 2 to 3 orders9.
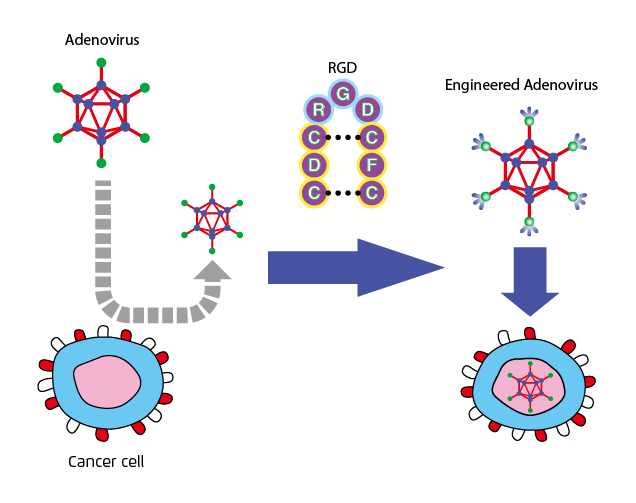
Figure 2. Insertion of RGD motif into adenoviruses
Arming OVs with genes that encode molecules enhancing the oncolytic potency is the basis of the engineering process. Sova et al (2004) modified another oncolytic adenovirus to express the TNF-related apoptosis-inducing ligand (TRAIL) leading to enhanced apoptotic cell death in animal tumour models10. In addition to inducing direct lysis of cancer cells, OVs also utilise other anticancer mechanisms to eradicate tumours whereas initiation of the host anti-tumour immune response is one of the most common mechanisms (Figure 1). Further, OVs trigger an antiviral immune activation in tumour cells, even without productive replication, that helps to activate anti-tumour immune stimulation11.
Safety of OVs primarily relates to the toxicity of the administration and environmental shedding of the infectious viruses. In the case of herpes simplex virus type 1 (HSV-1), which was one of the first viruses to be developed into an oncolytic agent, viral genes that are essential for viral replication in non-dividing cells are deleted. In addition, immune stimulatory genes boosting local cytotoxic immune responses are incorporated in oncolytic HSV-1 in order to enhance oncolytic efficacy, which often co-incidentally improve safety. Besides, improvement on targeting specificity would help limiting virus infection to only tumour cells12.
Established Efficacy of Virotherapy
The clinical efficacy of OV has been demonstrated in various clinical trials. For instance, in a recent phase-1 trial by Lang and co-workers, a single intra-tumoural injection of DNX-2401 – a tumour-selective, replication-competent oncolytic adenovirus – into biopsy-confirmed recurrent glioma was given to 37 patients. The results reflected that 20% of the patients survived longer than 3 years from treatment, and 3 patients had more than 95% reduction in the enhancing tumour (Figure 3), with all 3 of these dramatic responses resulting in more than 3 years of progression-free survival (PFS) from the time of treatment13.
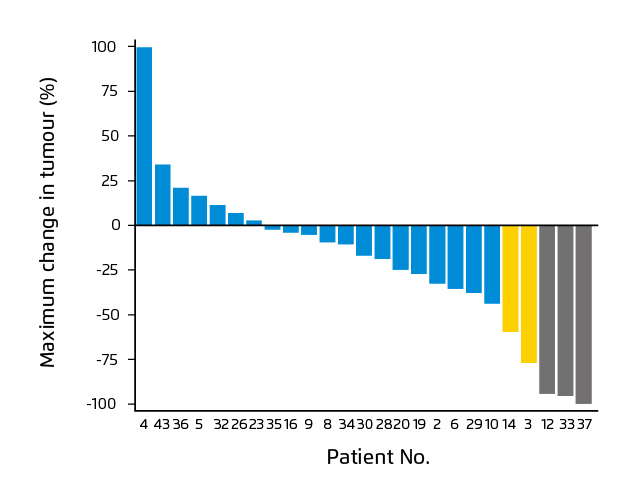
Figure 3. Waterfall plot showing maximal change in tumor size13
Besides as a monotherapy, previous preclinical and early-phase clinical trials have shown that combining OVs with other treatment options would yield clinical benefits. Speranza et al (2018) showed in a mice glioblastoma model that intra-tumoural injection of combined anti–programmed cell death protein 1 (PD-1) and a prodrug metabolising non-replicating adenovirus (AdV-tk) would increase intra-tumoural T-cell infiltration and result in a higher percentage of long-term survivors as compared to single treatment. Further, the results suggested that anti-PD-1 treatment improved the effectiveness of OV treatment by overcoming interferon-induced PD-L1–mediated inhibitory signals, while OV improved anti-PD-1 efficacy by increasing tumour-infiltrating T-cell activation14.
Future Perspectives of Oncolytic Virotherapy
There is emerging evidence on the therapeutic efficacy of OVs whereas the approval of T-VEC highlighted the clinical significance of virotherapy in cancer treatment. Nonetheless, further investigations in virus engineering for optimising the efficacy and safety of OVs will be essential for the advancement of virotherapy. Moreover, preclinical and clinical trials evaluating the appropriate OV treatment protocol, such as monotherapy or combined treatments, are crucial for improving the outcomes of oncolytic virotherapy as well.
References
1. Bierman et al. Cancer. 1953;6(3):591-605. 2. Ott et al. Clin Cancer Res. 2016;22(13):3127-3131. 3. May et al. Oncol Lett. 2019;18(5):5534-5542. 4. Zeng et al. Oncol Rep. 2013;29(3):1108-1114. 5. Koske et al. Int J cancer. 2019;145(7):1958-1969. 6. Davola et al.Oncoimmunology. 2019;8(6):e1581528. 7. Maroun et al. Future Virol. 2017;12(4):193-213. 8. Jhawar et al. Front Oncol. 2017;7(SEP). 9. Dmitriev et al. J Virol. 1998;72(12):9706-9713.10. Sova et al. Mol Ther. 2004;9(4):496-509. 11. Gujar et al. Trends Immunol. 2018;39(3):209-221. 12. Buijs et al. Hum Vaccines Immunother. 2015;11(7):1573-1584. 13. Lang et al. J Clin Oncol. 2018;36(14):1419-1427. 14. Speranza et al. Neuro Oncol. 2018;20(2):225-235.