
Diabetes and Cardiovascular Disease: An Evil Pairing
Currently, the prevalence of diabetes mellitus (DM) is approximately 463 million, which is equivalent to 9.3% of the world’s population. The global pandemic of DM is expected to rise, and this figure is expected to rise to 578 million (10.2%) by the year 20301. Of note, nearly 10% of the global health expenditure is spent on diabetic care, which equates to around $760 billion US dollars in 2019 and is expected to reach around US$845 million by the end of 20451. In fact, DM is the eighth leading cause of death and the third leading cause of years lost with disability. Furthermore, DM also increases the risk of cardiovascular disease (CVD), which includes ischaemic heart disease, stroke, and heart failure (HF). Together with DM, CVD contributes to a major diabetes-associated morbidity and mortality1. In addition, the healthcare cost related to DM management is predominantly attributed to the expenditure for treating these complications. Therefore, preventative measures are often deployed to reduce morbidity, mortality, and healthcare expenditures1.
The term diabetic heart disease (DHD) is coined from the fact that cardiac disease develops as a direct consequence of DM (type 1 diabetes [T1D] or type 2 diabetes [T2D])1. Moreover, DHD is a conglomeration of coronary artery disease (CAD), cardiac autonomic neuropathy (CAN), and diabetic cardiomyopathy (DCM), all of which confer molecular, structural, and functional changes in the cardiomyocytes1. Considering DM and obesity are closely associated, individuals with these two comorbidities are at an increased risk of developing CV-related morbidities and mortality since they increase the risk of vascular stiffness and accelerate atherosclerosis, which inadvertently leads to premature CVD and death in obese individuals2. Recently, semaglutide, a glucagon-like peptide 1 (GLP-1) analogue, has shown not only to improve the haemoglobin A1c (HbA1c), also known as glycated haemoglobin in diabetics, but also reduce the body weight in overweight and obese individuals with and without background DM3. Hence, semaglutide became synonymous with being the best-selling anti-diabetic medication with weight-reduction properties. However, this is about to change since a very recent real-world study suggested semaglutide’s potential beyond obesity, as well as glycaemic control as the injectable form of semaglutide has shown to confer CV protection by controlling atherogenic lipoproteins and hepatitis steatosis in obese individuals4.
Beat the Metabolic Dysregulation with Semaglutide
As we know, obesity is becoming more common as the global economy develops, and the existing obesity treatment remains inadequate5. Furthermore, obese individuals often have metabolic abnormalities creating excessive lipid accumulation, which along with increased levels of inflammation and oxidative stress, causing organ damage5. These changes inadvertently leads to disruption of the metabolism within the cardiac tissues affecting cardiac function, thereby increasing the cardiac load, which then causes morphological changes within the heart5. But what role does semaglutide play within CVD? Interestingly, animal studies demonstrated that semaglutide substantially alleviates obesity-induced lipid metabolism abnormalities, which then improves the cardiac ventricular wall thickening, thereby leading to a significantly lower myocardial collagen content in obese mice5. However, the questions arise on whether these beneficial effects are also prevalent in human subjects. The STEP 1 and 4 were phase III, 68-week placebo-controlled trials which included 1,961 participants in STEP 1 and 803 participants in STEP 4 trials, randomised to once-weekly subcutaneous injection (SC) of semaglutide 2.4 mg combined with lifestyle intervention or placebo. These trials evaluated the effects of once-weekly subcutaneous semaglutide 2.4 mg on cardiometabolic risk factors in overweight/obese without DM. Of note, most participants had one or more complications and comorbidity at baseline, with dyslipidaemia and hypertension6.
Results from the STEP 1 trial demonstrated a reduction in waist circumference and other metabolic parameters, including systolic blood pressure (SBP), diastolic blood pressure (DPB), fasting plasma glucose (FPG), fasting serum insulin (FSI), lipids (Figure 1) and homeostatic model assessment for insulin resistance (HOMA-IR) were greater in semaglutide group compared to the placebo group (p ≤0.001). Reductions in SBP, non-high-density lipoprotein (non-HDL) cholesterol, low-density lipoprotein cholesterol (LDL-C) and FPG were generally greater with semaglutide than placebo6. Similarly, in the STEP 4 trial, improvements in waist circumference, SBP, FPG, FSI, and lipids during the semaglutide run-in (week 0-20) were maintained over week 20-68 with continued semaglutide but deteriorated following the switch to placebo (p <0.001 [week 20-68])6. The net reductions in antihypertensive and lipid-lowering medication use occurred with the semaglutide group in both trials6.
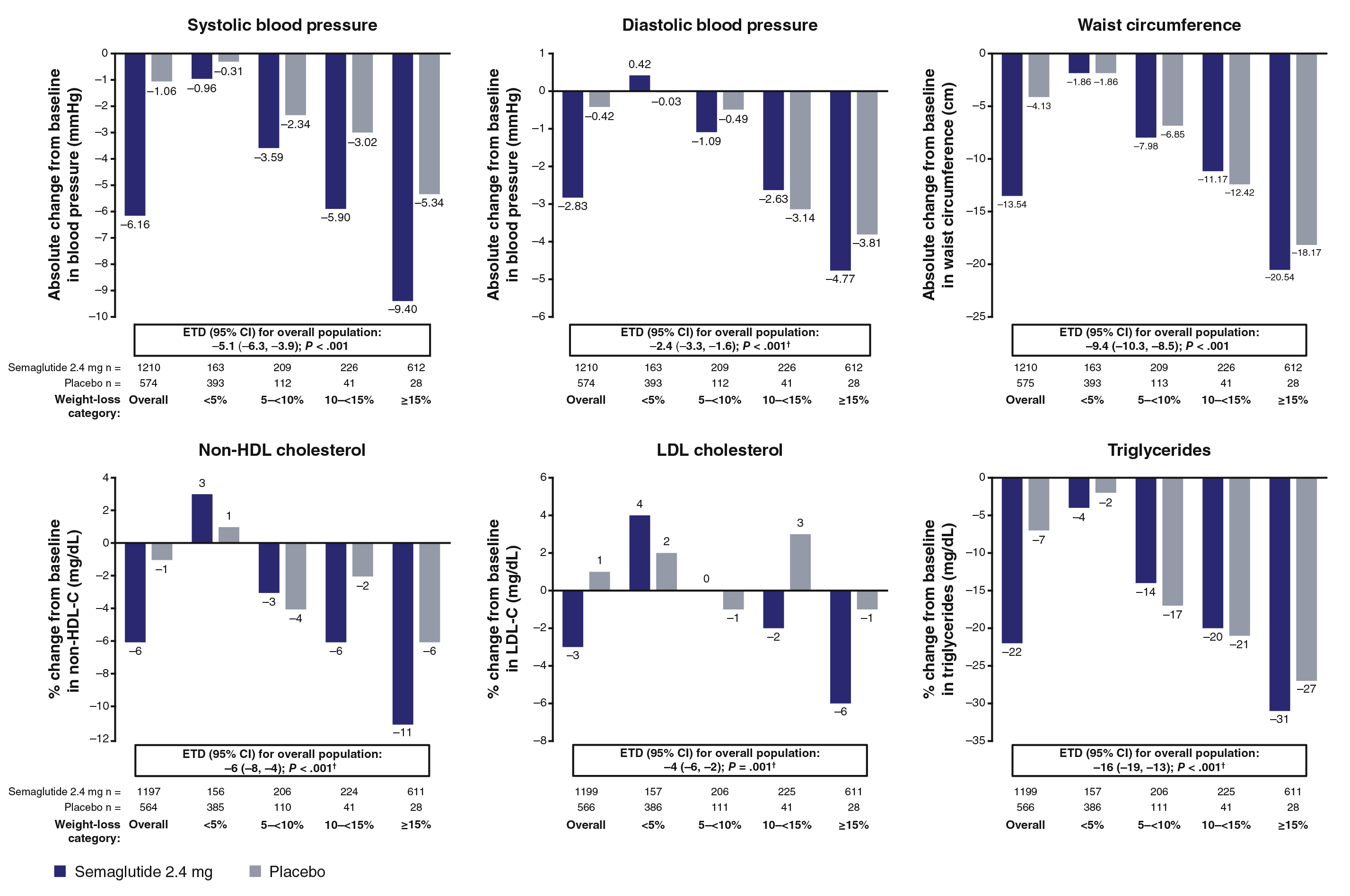
Figure 1. Change from baseline to week 68 in cardiometabolic risk factors in the overall population and by categorical weight loss in STEP 16
Similar findings were reported by a retrospective observational study performed by Folco et al., (2022) evaluating the impact of once-weekly SC injection of semaglutide on different endpoints indicative of metabolic control, CV risk, dietary behaviour, and treatment satisfaction among T2D. 104 patients with a mean age of 63.6± 10.4 years were treated with semaglutide7. Astonishingly, at 32 weeks, the HbA1c levels were reduced by 1.38%, FBG by -56.53 mg/dL, and weight by 6.03 kg. All the metabolic parameters (SBP, DBP, total cholesterol, HDL-C, LDL-C and non-HDL-cholesterol, and triglycerides) significantly improved. In addition, the use of glucose-lowering and antihypertensive treatment was also reduced. The study concluded that semaglutide conferred beneficial effects on the metabolic profile and multiple CV risk factors7. Therefore, beyond obesity control, semaglutide is also considered to be effective in reducing the cardiometabolic effects in individuals at risk of developing CVD.
Semaglutide Lowers the Highway to Cardiovascular Events
The microvascular complications of T2D have been progressively linked to an increased risk of macrovascular complications8. It is believed that T2D induce changes in endothelial structure and function within the micro- and macrovascular, increases the production of reactive oxygen species (ROS), augments mitochondrial superoxide release, and changes in protein kinase C metabolism. Furthermore, microvascular complications have been linked to an increased risk of HF in diabetics8. Considering that endothelial dysfunction in the large vessels promote atherogenesis, obesity-induced microvascular endothelial dysfunction causes organ perfusion impairment, thereby induces ischaemic heart disease, chronic kidney disease (CKD), exercise intolerance, and exacerbates the cognitive decline in aging. Weight reduction has been shown to improve microvascular endothelial function and may improve microvascular homeostasis9. Thereby, treatment related to weight reduction may help reduce the microvascular burden in obese individuals; this was demonstrated in an analysis of two randomised, double-blind CV outcome trials (LEADER and SUSTAIN 6), which recruited 12,637 (LEADER and SUSTAIN 6 trials) patients with T2D. Around 3,835 patients in LEADER (41%) and 1,640 in SUSTAIN 6 (50%) had a history of both microvascular and macrovascular disease. The post-hoc analysis examined the efficacy of two glucagon-like peptide-1 receptor agonist (GLP-1RA), liraglutide (1.8 mg) and once weekly semaglutide (0.5-1.0 mg). Of note, patients with microvascular disease were shown to have an increased risk of major adverse cardiovascular events (MACEs) compared to patients without microvascular disease. The results from the analysis suggested that both liraglutide and semaglutide reduced the CV outcomes compared to placebo in patients with a history of microvascular disease8. But what are the mechanisms related to semaglutide induced cardioprotection? The precise mechanisms behind semaglutide-induced cardioprotection remain elusive but is believed to be related to metabolic profile improvements.
Patti et al., (2023) performed a retrospective observational real-world study to determine the protective mechanisms of semaglutide in clinical practice, considering there had been strong preclinical evidence that supported the CV benefits of semaglutide through an effect on atherosclerosis. A total of 40 patients were included in the study, with 32% of subjects being habitual smokers and 85% suffering from hypertension4. In total, 85% of patients were on metformin therapy, and 12% of the total were on insulin treatment. The primary aims were the assessment of carotid intima-media thickness (cIMT) and Hb1Ac levels4. The secondary aims were the evaluation of anthropometric, glycaemic, and hepatic parameters and plasma lipids, including the assessment of the triglyceride/HDL ratio as an indirect marker of atherogenic small, density low-density lipoprotein (sdLDL) particles. The results obtained from the study showed that the SC injections of semaglutide lowered the HbA1c, cIMT, and metabolic profile (triglyceride/HDL ratio)4. Therefore, GLP-1RAs such as semaglutide may improve the metabolic profile, which may reduce the background inflammation and endothelial dysfunction10, which may then confer the CV benefit11. Thus, the real potential of semaglutide is undeniably life-changing for obese patients regardless of their DM status, since obesity is a direct risk factor for CVD12.
Semaglutide, A New Game-Changer in the Management of HF
The prevalence of heart failure with preserved ejection fraction (HFpEF) is increasing globally, and treatment for HFpEF remains elusive13. Approximately 60% of patients with HFpEF have the obesity phenotype characterised by a greater symptom severity, poorer exercise capacity, more adverse haemodynamic and greater risk for HF hospitalisation (HHF) than those with HFpEF without obesity13. STEP-HFpEF trial was a randomised controlled trial (RCT) that assigned 529 patients with HFpEF with a body mass index (BMI) of 30 or above to either once-weekly semaglutide (2.4 mg) or placebo for 52 weeks. The dual primary endpoints were the change from baseline in the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) and the change in body weight. Surprisingly, the mean changes in the KCCQ-CSS were 16.6 points with semaglutide and 8.7 points with placebo14. Moreover, patients also had greater functional capacity as the mean change in the 6-minute walk distance was 21.5 m with semaglutide and 1.2 m with placebo (estimated difference, 20.3 m; 95% confidence interval [CI]: 8.6-32.1; p<0.001). What about the inflammation? Does semaglutide affect obesity-related inflammation, which is also seen in HF patients? The answer to this question is yes, as the mean percentage change in the C-reactive protein (CRP) level was -43.5% with semaglutide and -7.3% with placebo (estimated treatment ratio, 0.61; 95% CI: 0.51-0.72; p<0.001)14. Notably, more participants reported serious adverse events in the placebo group compared to the semaglutide group (26.7% vs 13.3%, respectively)14.
The benefits of semaglutide is undeniably much larger than those seen in other pharmacological therapies used to treat patients with HFpEF. In fact, approximately 8-point estimated mean treatment differences in the KCCQ-CSS were noted with semaglutide compared to placebo, which is substantially 2 to 4-fold higher than that of other global trials on other pharmacological therapies (Figure 2)15. For instance, in the EMPEROR-Preserved trial, the least-squares mean differences in the KCCQ-CSS between the empagliflozin and placebo groups at 52 weeks was 1.5 points (95% CI: 0.64-2.36). Similarly, in the EMPERIAL-Preserved trial, the difference in the KCCQ-CSS was 0.32 points (95% CI: -3.07-3.71) at 12 weeks between the empagliflozin versus placebo-treated patients15.
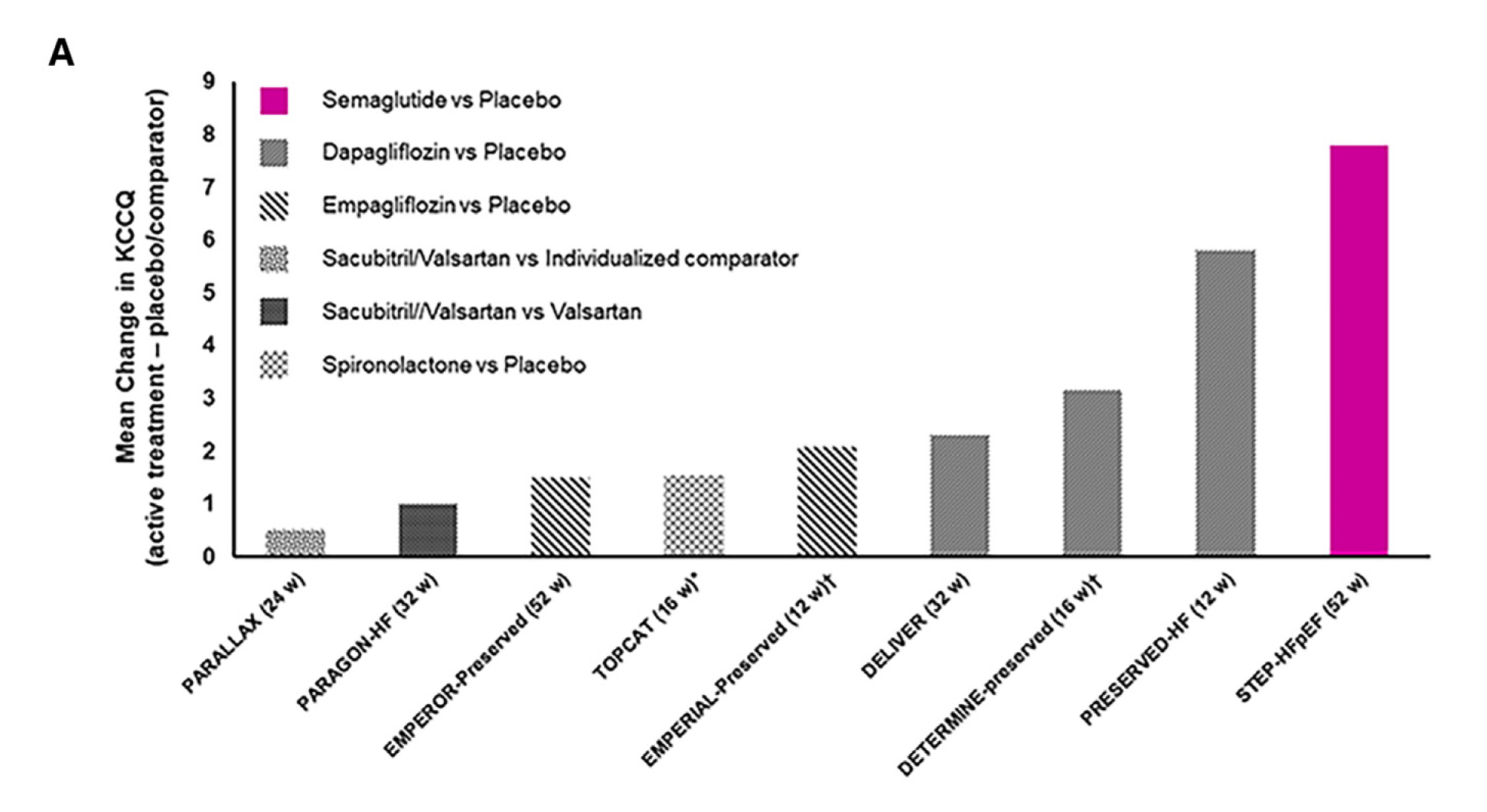
Figure 2. Comparisons of KCCQ(-CSS) effects in STEP-HFpEF and other HFpEF trials. Magnitude of benefits on the KCCQ(-CSS) in HFpEF trials15.
Semaglutide trial (STEP-HFpEF) also showed the largest number of wins in hierarchal composite endpoint and clinically important changes (Figure 3)15. Considering the current guidelines advocated using either the sodium-glucose cotransporter-2 inhibitors (SGLT2i) or GLP-1RAs such as semaglutide for treating patients with T2D and atherosclerotic cardiovascular disease (ASCVD), prioritising the combined treatment routinely in this population remains unclear. Whether the protection from CV outcomes in individuals with T2D is greater with the combined use of SGLT2i and GLP-1RA (vs either treatment alone) remains debatable16. In the post-hoc analysis of the Harmony Outcomes involving 9,462 participants, 575 were treated with SGLT2i at baseline and assigned to albiglutide, showed a reduction in composite CV death, myocardial infarction, or stroke (MACEs) with or without SGLT2i (p=0.70)16. A further meta-analysis of Harmony Outcomes and AMPLITUDE-O included 13,538 patients, of whom 1,193 used SGLT2i. Compared to placebo, GLP1-RAs reduced MACEs independent of the SGLT2i use (hazard ratio [HR]: 0.77; 95% CI: 0.68-0.87 without SGLT2i; and HR: 0.78; 95% CI: 0.49-1.24 with SGLT2i) (p = 0.95)16. The conclusion drawn from this analysis suggested that GLP-1RA reduces the risk of adverse CV events, independent of the background SGLT2i use, without an increased risk of serious adverse events16. These revelations further support the use of GLP-1RA, which offers CV benefits in patients with T2D, regardless of their previous SGLT2i status16.
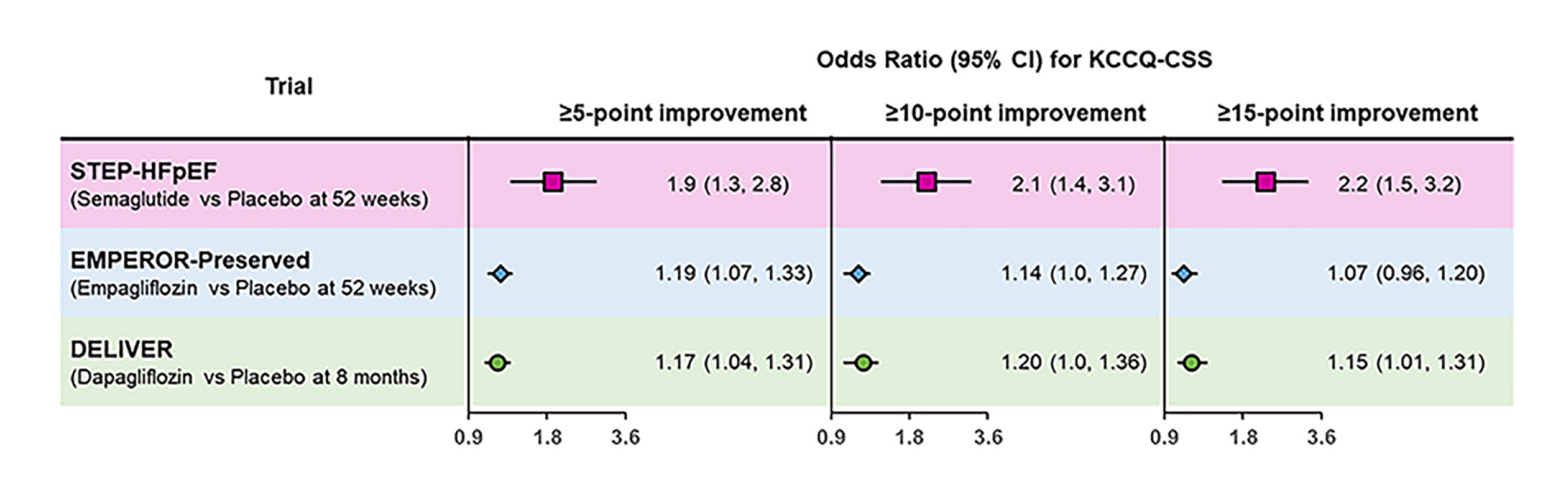
Figure 3. Responder-analysis results for the KCCQ-CSS (≥5-, ≥10-, and ≥15-point improvements) in the STEP-HFpEF, EMPEROR-Preserved, and DELIVERY trials15.
References
1. ORajbhandari J,et al. World J Diabetes 2021; 12(4): 383-406. 2. Brown OI, et al. Diabetes Care 2023; 46(8): 1531-40. 3. Wilding JPH, et al. N Engl J Med 2021; 384(11): 989-1002. 4. Patti AM, et al. Biomedicines 2023; 11(5). 5. Pan X, et al. J Inflamm Res 2022; 15: 6409-25. 6. Kosiborod MN, et al. Diabetes Obes Metab 2023; 25(2): 468-78. 7. Di Folco U, et al. Acta Diabetologica 2022; 59(10): 1287-94. 8. Verma S, et al. Diabetes Obes Metab 2020; 22(11): 2193-8. 9. Csipo T, et al. Geroscience 2018; 40(3): 337-46. 10. Pan X, et al. International Immunopharmacology 2023; 119: 110196. 11. Candido R, et al. J Clin Med 2023; 12(18). 12. Cercato C, et al. Diabetology & Metabolic Syndrome 2019; 11(1): 74. 13. Borlaug BA, et al. Nature Medicine 2023; 29(9): 2358-65. 14. Del Pozo Cruz B, et al. JAMA Neurol 2022; 79(10): 1059-63. 15. Verma S, et al. Cell Metabolism 2023; 35(10): 1681-7. 16. Neves JS, et al. Journal of the American College of Cardiology 2023; 82(6): 517-25.





