

Editor-in-Chief, V·Pulse

Doctor at the National Health Services (NHS), United Kingdom (UK)
Renal cell carcinomas (RCCs) are the most common type of kidney cancer in adults and is prevalent in adult male aged between 60 and 70. Notably, clear cell RCC (ccRCC) is the most abundant subtype of RCC with the worse disease-specific survival as it tends to be diagnosed at a more advanced stage1. Apart from the mortality risk, RCC also poses significant psychological distress on patients2. Therefore, sensitive tools facilitating early diagnosis of RCC and therapies effectively countering the disease are undoubtedly remains paramount for improving patient survival outcomes.
How Common is RCC?
RCC is the most common type of kidney cancer in adults, accounting for about 80% to 85% of all primary renal neoplasms. The global incidence of RCC varies, with the highest rates observed in the Czech Republic and North America3. Of note, the incidence of RCC has gradually increased as a result of improved cross-sectional abdominal imagining available4.
Notably, the mortality rate of RCC has been reported to be approximately 2% of all cancers in 20164. According to a cancer report published by the Hong Kong Hospital Authority in 2021, kidney cancer was the seventh most common malignancy reported in the local male population, with a crude incidence rate of 17.3 cases per 100,000 population. Remarkably, a gradual increase in the local incidence of kidney cancer has been observed over the recent years (Figure 1)5.

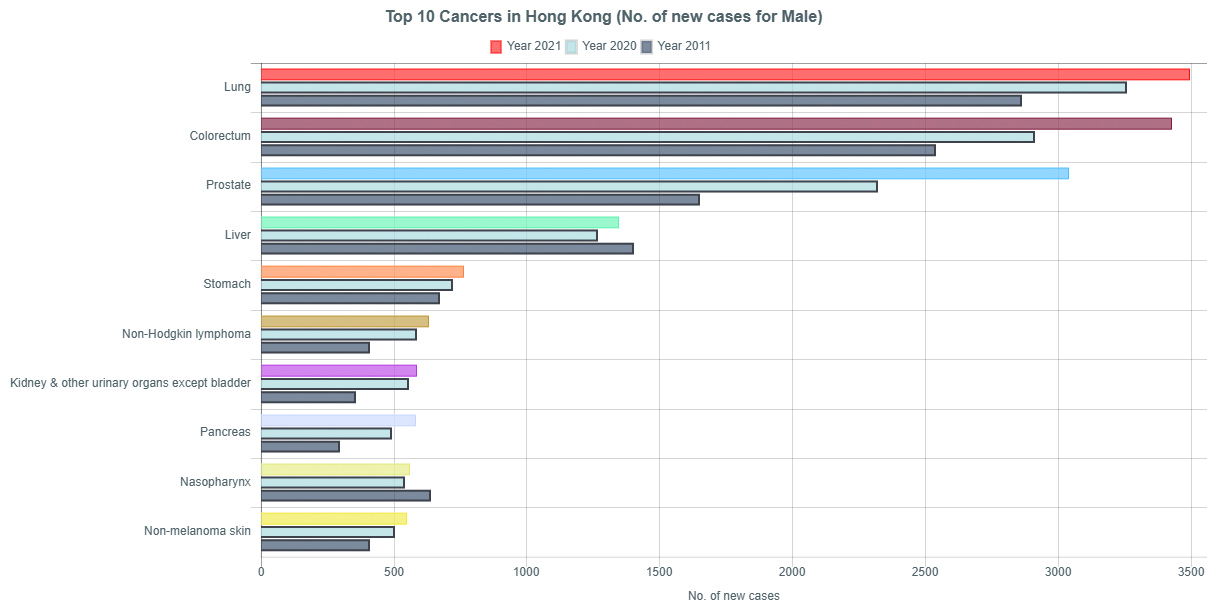
Figure 1. Top 10 cancers in local male population in 2011, 2020, and 20215
The Classification of RCC
While RCC is the predominant type of kidney cancer, the disease is heterogeneous and may originate from different cells throughout the nephrons. The World Health Organization (WHO) classified the RCC into different subtypes according to its morphologic, molecular, and genetic features. Briefly, RCC can be classified into clear cell and non-clear cell subtypes, and the non-clear cell RCC can be further subdivided into papillary, chromophobe, and other rarer forms, such as collecting duct carcinoma6. Both ccRCC and papillary RCC originate from the proximal convoluted tubules (PCT), while chromophobe RCC arises from the distal connecting tubules (DCT) and collecting duct system, particularly the intercalated cells4.
ccRCC has a worse prognosis compared to the papillary or chromophobe RCC. In contrast, papillary RCC is characterised by slow growth and divided into type I and type II based on its histological and genetic differences. Both types of papillary RCCs have sporadic and hereditary forms. Type I papillary RCC can be detected at an early stage, hence carries a better prognosis4. Chromophobe RCC is usually sporadic and is common in the sixth decades of life with a good prognosis6.
Are You at Increased Risk of RCC?
Despite being the most common type of kidney cancer, RCC is often known as a silent disease since patients often elicit no suggestive symptoms of malignancy at early stages, whereas about 30% of patients have metastatic disease at the time of diagnosis7. Most RCCs are found incidentally on the thoracoabdominal imaging for unrelated issues. Nonetheless, as the solid tumour grows, symptoms including haematuria, back pain, flank mass, fatigue, weight loss, anaemia, fever, and high serum calcium level may appear. Of interest, the presence of gross haematuria, flank pain, and a palpable abdominal mass is indicative of an advanced disease3.
The risk of RCC is associated with age, gender, and ethnicity, while males are often diagnosed with RCC at almost twice the rate of females, with a greater prevalence reported in the African males. Furthermore, most RCC cases are diagnosed between the ages of 60 and 701. Capitanio et al (2019) reported an association between smoking, obesity, and hypertension with the reported incidence of RCC8. In addition, existing literature suggested that chronic kidney disease (CKD), exposure to cadmium and trichloroethylene, consumption of red and processed meat, viral hepatitis, type-2 diabetes (T2D), and decreased physical activity are also associated with the increased risk of RCC4.
A Briefing on the Disease Burden of RCC
Given most RCC patients are diagnosed in advanced stages of the disease, the prognosis for long-term survival remains suboptimal, and the 5-year survival rate for patients with stage IV RCC ranges from 5% to 10%; a further 20% to 30% of patients with initially localised disease relapse after nephrectomy. A retrospective longitudinal study performed by Mantovani et al (2008) reported that the risk of death was significantly higher amongst metastatic-stage patients, with a median survival of about 6 months (Figure 2)9.
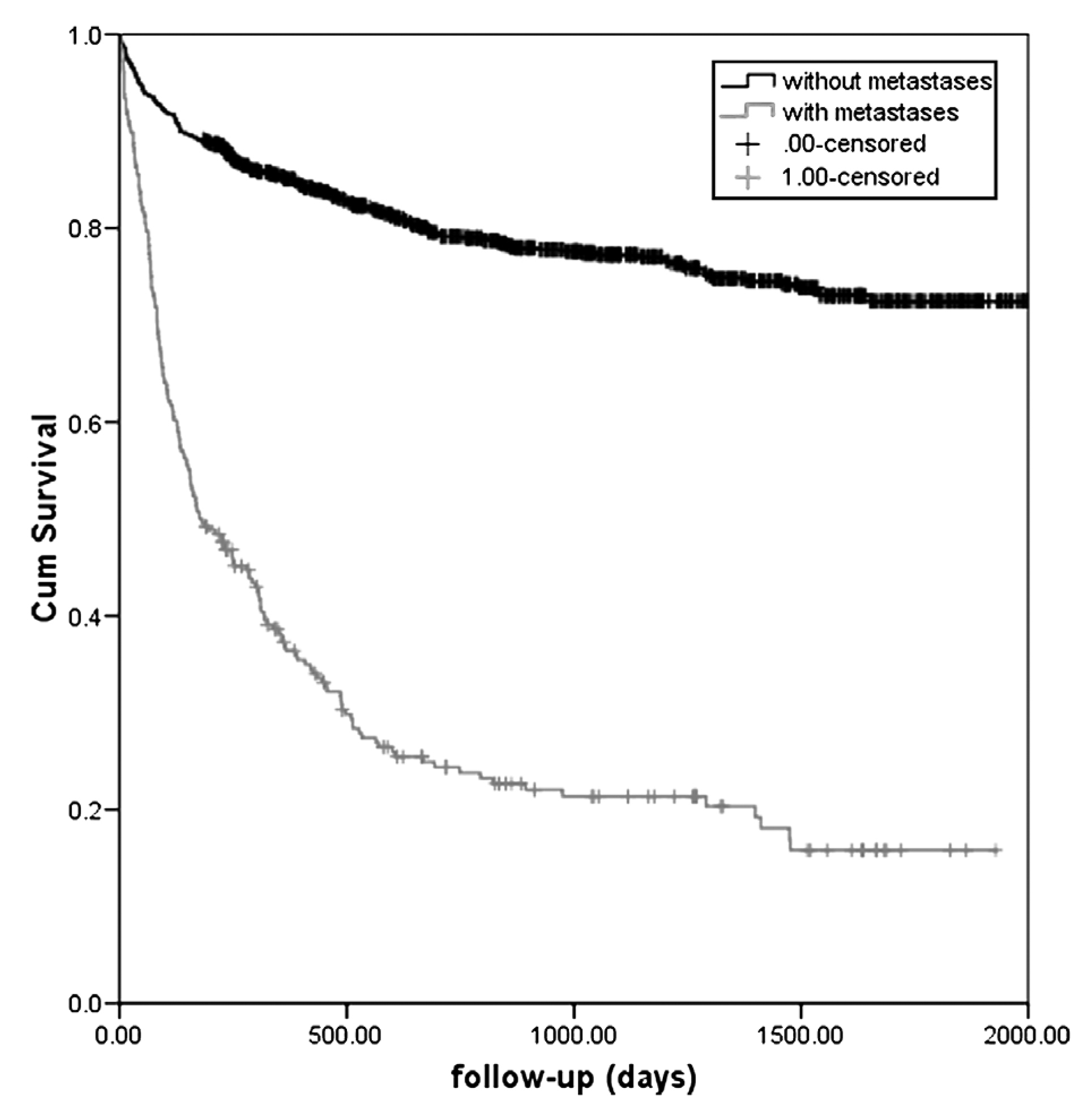
Figure 2. Kaplan–Meier survival curves of individuals with a diagnosis of RCC with and without metastases9
Mantovani’s study further estimated that the total healthcare costs per patient over the maximum follow-up (between 1996 and 2006) was €16,090 for the localised stage group and €17,656 for the metastatic stage group. Of note, most of the total costs estimated were attributed to hospital care9. The findings thus reflected the substantial epidemiologic and economic burden of RCC to the healthcare system and society.
Psychosocial Impacts - The Second Attack of Kidney Cancers
Apart from the pathological and socioeconomic burden, kidney cancers also impose significant psychosocial impacts on patients. For instance, a survey by Draeger et al (2018) assessed the psychosocial stress of 74 patients undergoing inpatient surgical or medical treatment for RCC. The survey identified the main stressors experienced by the patients, including anxiety (32%), pain (27%), nervousness (26%), sadness, worry and sleeping difficulties (20%). Importantly, 27% of patients were in need of psychosocial care, which was significantly higher than the self-reported need (7%, p<0.001). Moreover, the need for psychosocial care, as represented by the National Comprehensive Cancer Network Distress Thermometer (NCCN-DT), did not correlate with tumour stage, sex, or prognosis10. These results highlighted that the need for psychosocial care of RCC patients was often underreported and largely unrecognised by patients as well as clinicians.
The Clinical Management of RCC
The treatment of RCC depends on the stage and characteristics of the disease as well as the patient’s baseline health and preferences. Of importance, a shared decision making among the urologist, family physician, and the patient is recommended prior to treatment initiation1. Solid tumours are managed according to their size. Approximately 20% of the small kidney masses ultimately have a benign pathology, and their average growth rate is generally slow, approximately 2-3 mm/year. Therefore, small masses can be monitored with active surveillance (AS). Besides, active surveillance can also be applied in determining the rate of tumour growth of larger tumours4.
Early-stage RCCs confined to the kidney may require curative nephrectomy or partial nephrectomy. On the other hand, radiofrequency ablation (RFA) or cryotherapy is an option in patients with bilateral tumours and small cortical tumours. Imaging surveillance is an option in older adults with a short life expectancy who are not for any surgical interventions3.
While advanced-stage RCC remains incurable, palliative treatments are often considered3. In 2022, the Hong Kong Urological Association and the Hong Kong Society of Uro-Oncology jointly addressed the consensus recommendations for managing advanced and metastatic RCC. The panel advocated that the International Metastatic RCC Database Consortium (IMDC) risk category remained a key consideration in deciding treatment strategy for metastatic ccRCC7.
For IMDC favourable-risk advanced or metastatic ccRCC, tyrosine kinase inhibitor (TKI) monotherapy was regarded as the preferred first-line systemic therapy. In IMDC intermediate/poor-risk cases, immune checkpoint inhibitor (ICI)-based combination treatment is recommended (Figure 3A). Provided the standard of care for metastatic non-clear cell RCC remains unclear, the panellists favoured TKI monotherapy based on existing evidence. Notably, the panellists addressed that the use of adjuvant systemic treatment after nephrectomy was dependent on patient preference after weighing the benefits and risks. For subsequent treatment after progression on first-line systemic treatment, in patients who demonstrated progression after ICI-based combination treatment, TKI monotherapy is recommended by the panel. In contrast, ICI-based combination treatment is favoured for disease progression after TKI monotherapy (Figure 3B)7.
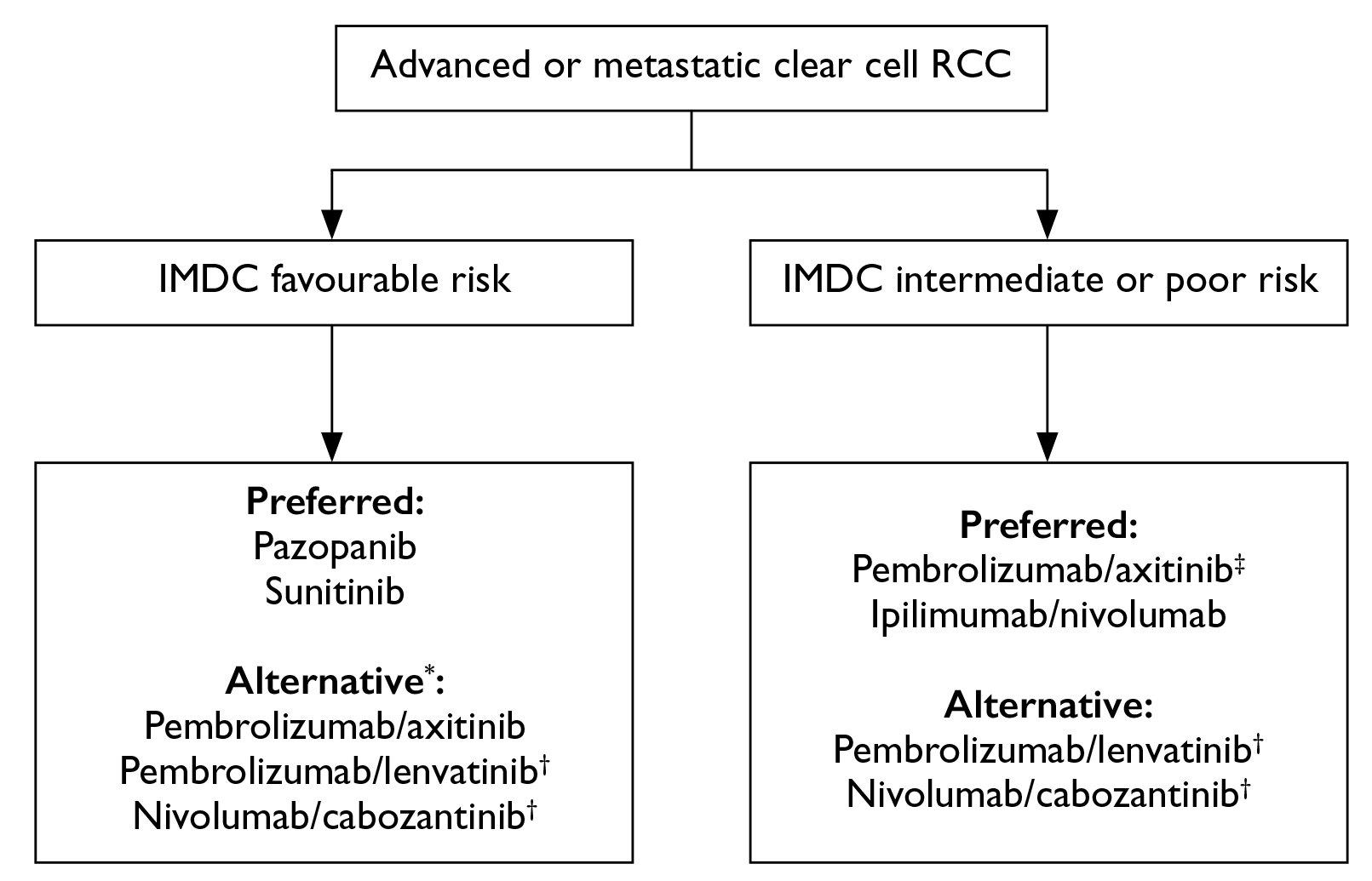
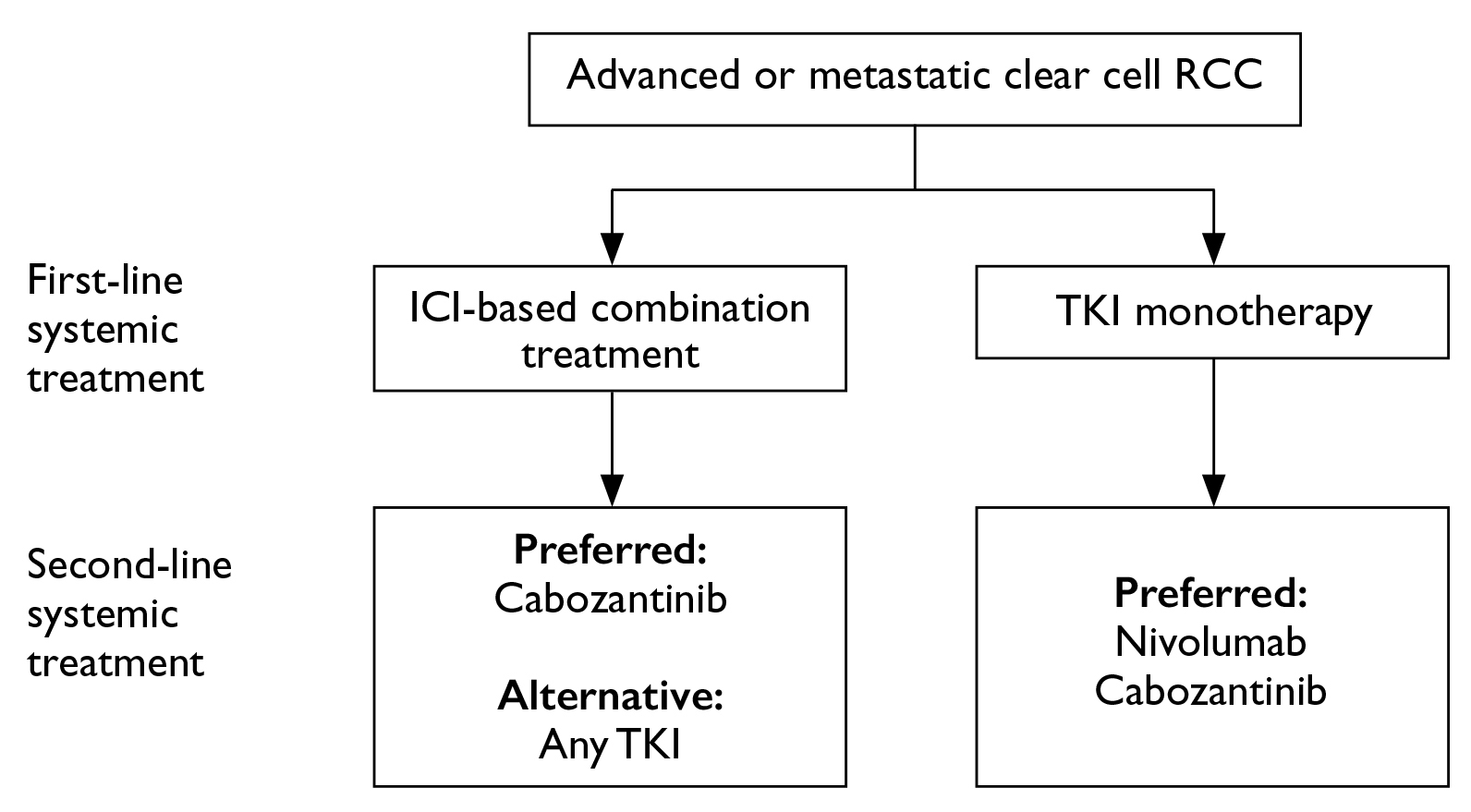
Figure 3. Expert panel recommendations of A) systemic first-line treatment regimens and B) second-line treatment regimens for advanced or metastatic clear cell renal cell carcinoma7
The Development of Liquid Biopsy in Detecting RCC
Undoubtedly, late diagnosis and the lack of effective disease surveillance as well as suboptimal drug efficacy prediction methods hinder the optimal outcome of RCC patients. Thus, developing a minimally invasive screening tool and identifying appropriate biomarkers for RCC is highly desirable. In this regard, the concept of liquid biopsy has attracted the attention of researchers and clinicians.
The technique of liquid biopsy involves collecting and analysing circulating tumour cells (CTCs) and other tumour markers, such as cell-free tumour DNA (ctDNA), cell-free RNA (cfRNA), exosomes, and tumour-derived metabolites in the blood, urine, pleural fluid, and ascites. Liquid biopsy has been widely used in various cancers, such as liver and bladder cancers. The technique facilitates early and differential diagnosis, prognostic prediction, therapeutic strategy selection, and real-time monitoring of treatment responses. Given their lower invasiveness, liquid biopsies allow continuous collection of samples from patients and can potentially be developed as a screening tool for RCC11.
A recent investigation by Sequeira et al (2022) demonstrated that RCC patients (n=124) showed significantly higher circulating levels of microRNAs (miRNAs), hsa-miR-155-5p, compared to healthy donors (n=64), whereas the opposite was observed for hsa-miR-21-5p levels (Figure 4A and 4B). Also, hsa-miR-21-5p and hsa-miR-155-5p panels detected RCC with high sensitivity (82.66%) and accuracy (71.89%). The hsa-miR-126-3p/hsa-miR-200b-3p panel identified ccRCC, with 74.78% sensitivity12. These results highlighted the potential of detecting RCC by analysing miRNA levels in plasma samples.
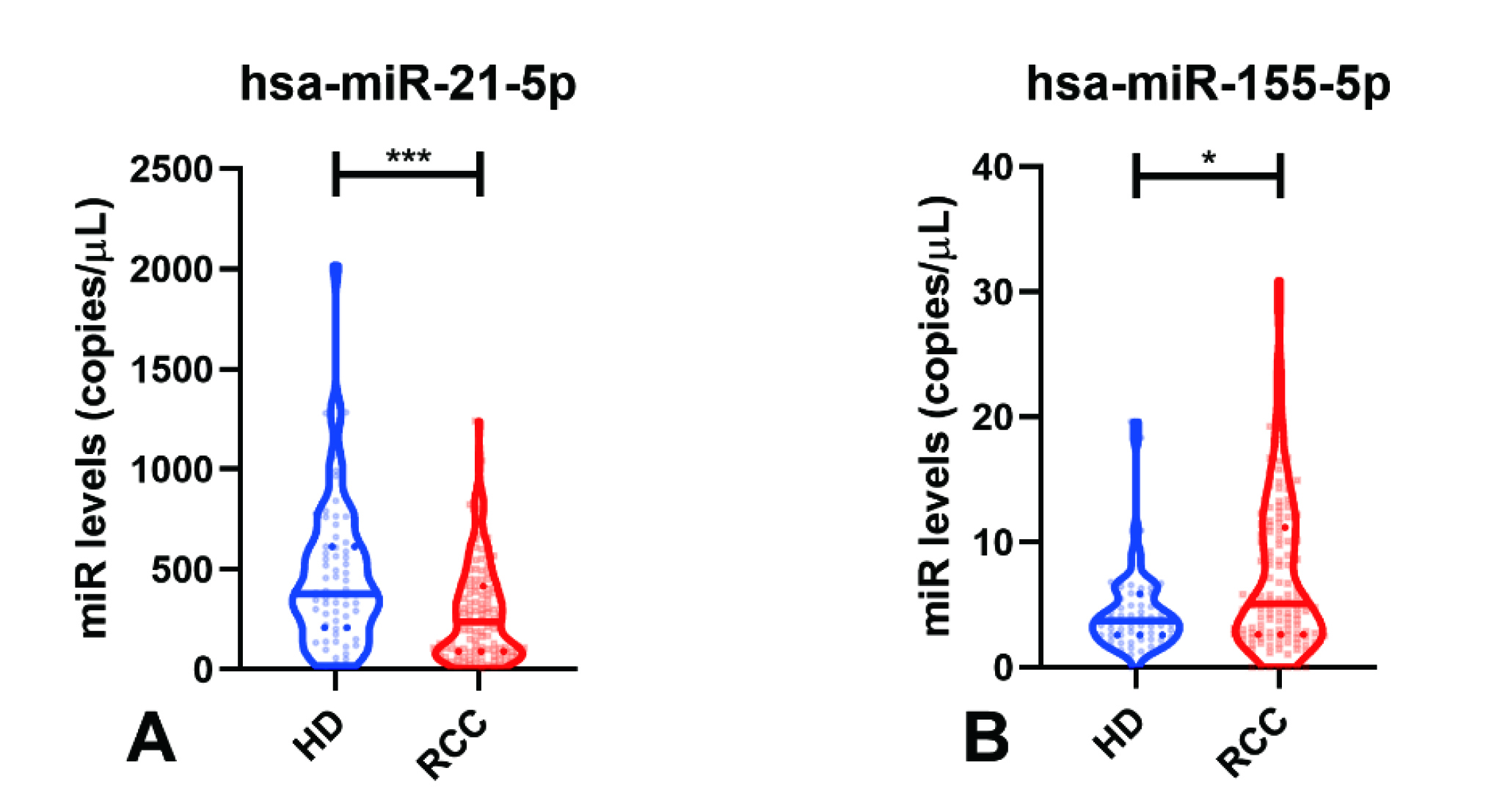 Figure 4. miRNA levels in Healthy Donors (HD) and RCC samples of A) hsa-miR-21-5p, and B) hsa-miR-155-5p12
Figure 4. miRNA levels in Healthy Donors (HD) and RCC samples of A) hsa-miR-21-5p, and B) hsa-miR-155-5p12
Emerging Therapies Combating RCC
Thanks to the advances in pharmacotherapies, effective agents countering RCC are emerging. For instance, in the phase III CLEAR trial (2021), the efficacy of lenvatinib in combination with pembrolizumab or everolimus against advanced RCC was compared with that of sunitinib. After a median follow-up of 26.6 months, the objective response achieved with lenvatinib plus pembrolizumab was 71%, whereas those achieved with lenvatinib plus everolimus and sunitinib were 53.5% and 36.1%, respectively13.
Patients who received lenvatinib plus pembrolizumab (n=355) exhibited a significantly longer median progression-free survival than sunitinib (PFS, 23.9 vs 9.2 months, p<0.001). Lenvatinib plus everolimus also yielded a significantly longer median PFS than sunitinib (14.7 vs 9.2 months, p<0.001, Figure 5). Moreover, 79.2% of the patients in the lenvatinib plus pembrolizumab group, 66.1% of the patients in the lenvatinib plus everolimus group, and 70.4% of the patients in the sunitinib group were alive at 24 months. Although the median overall survival (OS) was not reached with any treatment, the OS was longer with lenvatinib plus pembrolizumab than sunitinib (p=0.005). However, no significant improvement in OS was observed with lenvatinib plus everolimus compared to sunitinib (p=0.30)13.
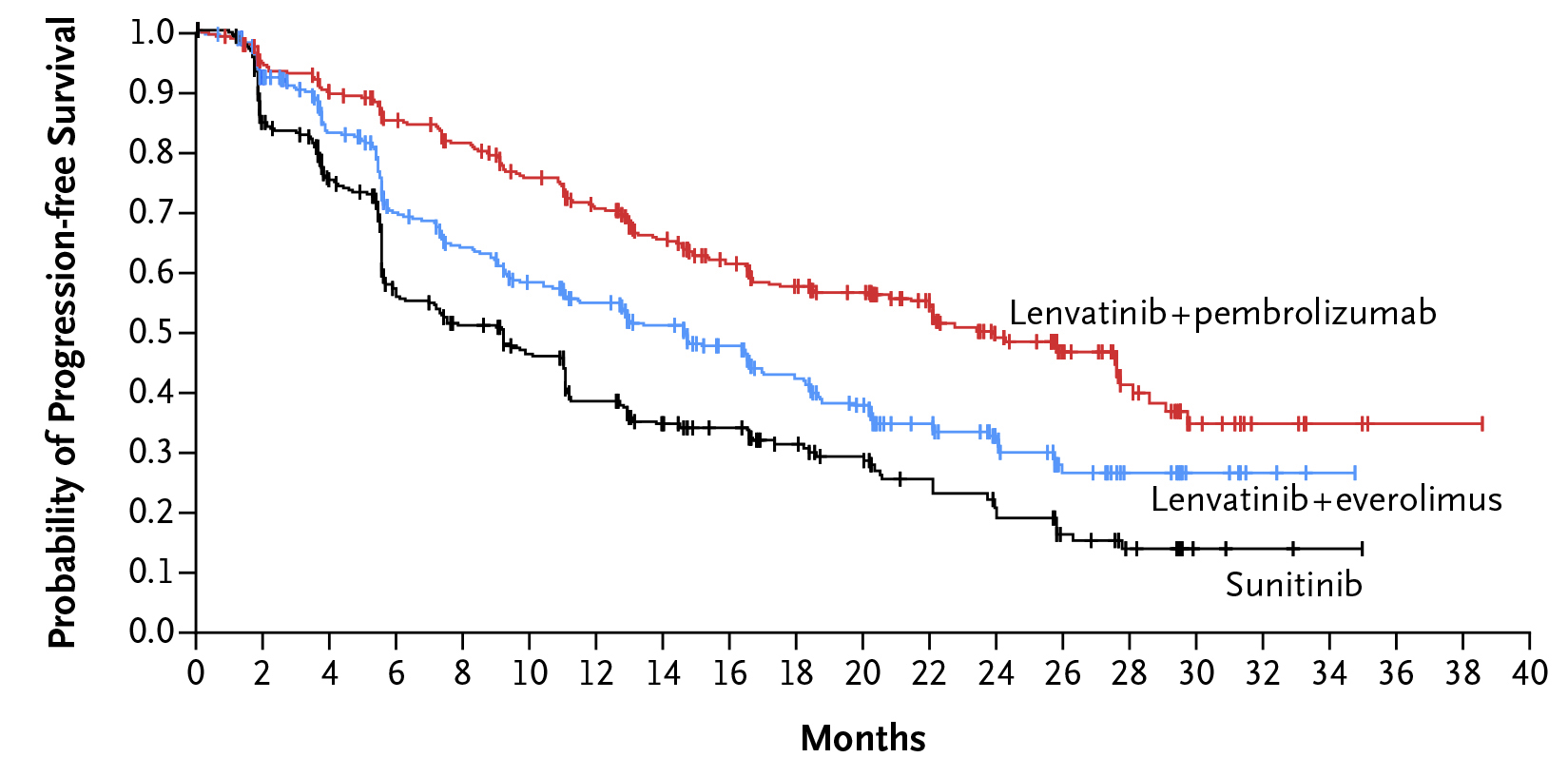 Figure 5. PFS achieved in the CLEAR trial13
Figure 5. PFS achieved in the CLEAR trial13
The safety profile of lenvatinib plus pembrolizumab was comparable with lenvatinib plus everolimus and was consistent with the known profile of each drug. Hypertension, diarrhoea, weight decrease, and proteinuria were the most common adverse events of grade 3 or above13. Hence, the results demonstrated that combining ICI and TKI is a promising treatment strategy for countering advanced RCC.
Triumph over RCC
RCC is the most common kidney cancer, which substantially impacts patient survival and psychosocial well-being. While early diagnosis of RCC is still clinically challenging, effort has been made to develop accurate and safe diagnostic tools, such as liquid biopsies, for detecting RCC at early stages. Given the psychosocial impact of RCC, an easy-to-use assessment can help identify patients in need and guide subsequent psychological support, and thus facilitating holistic patient care. Remarkably, the treatment landscape for advanced and metastatic RCC is evolving. More efficacious therapies, particularly ICI-based combination regimens, have been shown to offer promising survival benefits. With a better understanding of the biochemical, molecular, and histological characteristics of RCC, individualised treatment catered for a specific patient or groups of patients is made possible and is expected to shift the paradigm of treatment in the clinical management of RCC.
References
1. Gray et al. Am Fam Physician 2019; 99: 179–84. 2. Vartolomei et al. J Clin Med 2022; 11. DOI:10.3390/JCM11143944. 3. Garfield and LaGrange. Renal Cell Cancer. StatPearls 2023. 4. Bahadoram et al. Giornale Italiano di Nefrologia 2022; 39. 5. Hong Kong Cancer Registry, Hospital Authority. 6. Warren et al. World Journal of Urology 2018 36:12 2018; 36: 1913–26. 7. Poon et al. Hong Kong Med J 2022; 28: 475–81. 8. Capitanio et al. Eur Urol 2019; 75: 74–84. 9. Mantovani et al. European Journal of Cancer Supplements 2008; 6: 46–51. 10. Draeger et al. Ther Adv Urol 2018; 10: 175. 11. Li et al. Molecular Cancer 2023 22:1 2023; 22: 1–51. 12. Sequeira et al. Cancers (Basel) 2022; 14: 858. 13. Motzer et al. N Engl J Med 2021; 384: 1289–300.





