

Doctor at the National Health Services (NHS), United Kingdom (UK)

Doctor at Mater Dei Hospital,
Malta
Kawasaki disease (KD) is also known by the name as mucocutaneous lymph node syndrome and is an acute, self-limiting medium vessel vasculitis that has a predilection for the coronary arteries1. KD was first described in a 1967 report by Japanese paediatrician, Tomisaku Kawasaki. The cardiac sequela was later documented in 1970, following investigation of 10 autopsy cases of sudden cardiac death following a diagnosis of KD2. In fact, KD is the leading cause of acquired heart disease in developed nations and is slowly overtaking rheumatic heart disease (RHD) in developing countries1. The aetiology of KD remains elusive, and most likely involves a complex interplay between genetic susceptibility and various environmental triggers3. Recent studies have shown certain genetic markers (such as human leukocyte antigen-B51 [HLA-B51] and HLA-BW22J2 serotypes, chemokine receptor gene-cluster C-C chemokine receptor type 2 [CCR2]-CCR5 haplotypes and low affinity immunoglobulin gamma Fc region receptor III-A [FCGR3A] polymorphism of the immunoglobulin G [IgG] receptor IIIa) show a predisposition to the disease1.
Notably, the disease is most common in children below the age of 5, but can present at any age, even in adults. Furthermore, there is a slight male predominance (1.5:1 ratio for male to female, respectively). In addition, males are more likely to suffer from complications and death1. Closer to home, KD is mostly seen in children of Asian descent, particularly Japanese and, is least common in Caucasian children. There are approximately 10-20 in 100,000 children ages < 5 years in the United States (US) and Canada to 50-250 in 100,000 in Japan, Taiwan or Korea affected by the disease1. The incidence in Hong Kong is approximately 74 per 100,000 children < 5 years4. Due to the seriousness of the KD, the American Heart Association (AHA) 2017 guidelines on KD listed 21 other clinical findings affecting seven major organ system that were not previously described in the original case definition for KD3. To understand more regarding this complex disease, we have invited Dr. Debono Sant Cassia, one of the doctors at Mater Dei Hospital to discuss the clinical features of the disease, in addition to the importance of early diagnosis to prevent the risk of coronary artery aneurysm (CAA) in children.
Since the aetiology of KD is not known, it is hypothesised that there is likely an infectious agent which enters via the respiratory tract and activates the inflammatory cells (lymphocytes, cytokines and proteinases)1. So, what triggers the coronary arteritis? Dr. Debono Sant Cassia suggested that the oligoclonal immunoglobulin A (IgA) plasma cells may act as an initiators which predisposes individuals to coronary inflammation, and myocarditis1. Furthermore, activated immune cells also causes vascular lesions, most important the coronary aneurysms that occurs over weeks to months. This then leads to the thickening of coronary aneurysmal wall and allow stenosis or thrombus formation which inadvertently leads to the myocardial infarction, followed by rupture and ischaemia-related dysrhythmias or death1.
Dr. Debono Sant Cassia emphasised that children with incomplete KD (fewer than four out of five clinical characteristics) are at a higher risk since there is often a delay in treatment and the risk of developing coronary artery disease increases. Even though treatment reduces the likelihood of developing CAA, around 5% of patients may still go on to develop aneurysms despite treatment, making KD the leading cause of acquired heart disease in children in the United States, according to Dr. Debono Sant Cassia5. Asides from cardiac issues, patients are also at risk of developing Kawasaki shock syndrome (KSS), characterised by KD with associated systolic hypotension, or other signs of poor perfusion5. On this note, Dr. Debono Sant Cassia emphasised that KD should be managed by a multidisciplinary team consisting of a paediatrician, cardiologist, and an infectious disease specialist, in addition to a rheumatologist in selective cases especially when the diagnosis remains uncertain or when the illness remains unresponsive to treatment. To demonstrate the correlation between the delayed treatment and CAA risk, Dr. Debono Sant Cassia shared a study performed by Mossberg et al., (2020) that evaluated factors associated with the risk of developing CAA. The study analysed data from 77 children diagnosed with KD in Sweden and found 24 of them (31%) had CAA6. Interestingly, almost all children that developed CAA (95.8%) were treated with immunoglobulins but only 75% were treated within 10 days of symptoms debut. In children without CAA, 96.2% received immunoglobulin therapy, of which 90.6% were treated within 10 days of symptom debut6. The study emphasised the importance of early diagnosis and treating the disease early. Dr. Debono Sant Cassia stressed that the delay is often seen in patients with incomplete KD, and delaying the initiation of Ig treatment in these subset of patients is often detrimental since these patients often have a longer fever duration, and are younger than those with complete KD onset with a higher incidence of coronary artery disease7. Clinicians should suspect incomplete KD if a child has fever that lasts for more than 5 days, especially if the body temperature exceeds 38.5oC or remains as high as 39oC to 40oC, even after anti-infective treatment7. An echocardiography of the heart and great blood vessels should be performed in a timely manner and if imaging shows coronary artery involvement, or other large-vessel disease, a diagnosis of incomplete KD should be on top of the list, according to Dr. Debono Sant Cassia7.
Recently, the rapid spread of coronary virus 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has led to a global pandemic8. Paediatric COVID-19 rate were reported to be less than 3%, which is much less than that of adults. Nevertheless, since April 2020, several countries has seen an increase in multisystem inflammatory symptoms which included KD and these often-required intensive care unit admissions8. A link between COVID-19 infection and KD spike was highlighted by Ouldali et al., (2020), who evaluated the association between COVID-19 and the increased incidence of KD during the same period. The study included 230 patients with KD and reported a spike in KD incidences related to SARS-CoV-2 (6 per month; 497% increase [95% CI: 72-1082]; p= 0.0011), starting 2 weeks after peak of the COVID-19 epidemic8. The authors also reported noting a second peak which was observed in December 2009 (6 cases per month; 365% increase [31-719]; p=0.0053), concomitant with the influenza A haemagglutinin-1 neuraminidase-1 (H1N1), also known as swine flu pandemic (Figure 1)8. The study concluded that SAR-CoV-2 may trigger KD and was related to an increase incidence of KD8. In relationship to the findings from this study, Dr. Debono Sant Cassia suggested that we should remain vigilant during these epidemics and ensure we take the best diagnostic approach to help diagnose the disease as early as possible in order to reduce the disease burden in paediatric population.
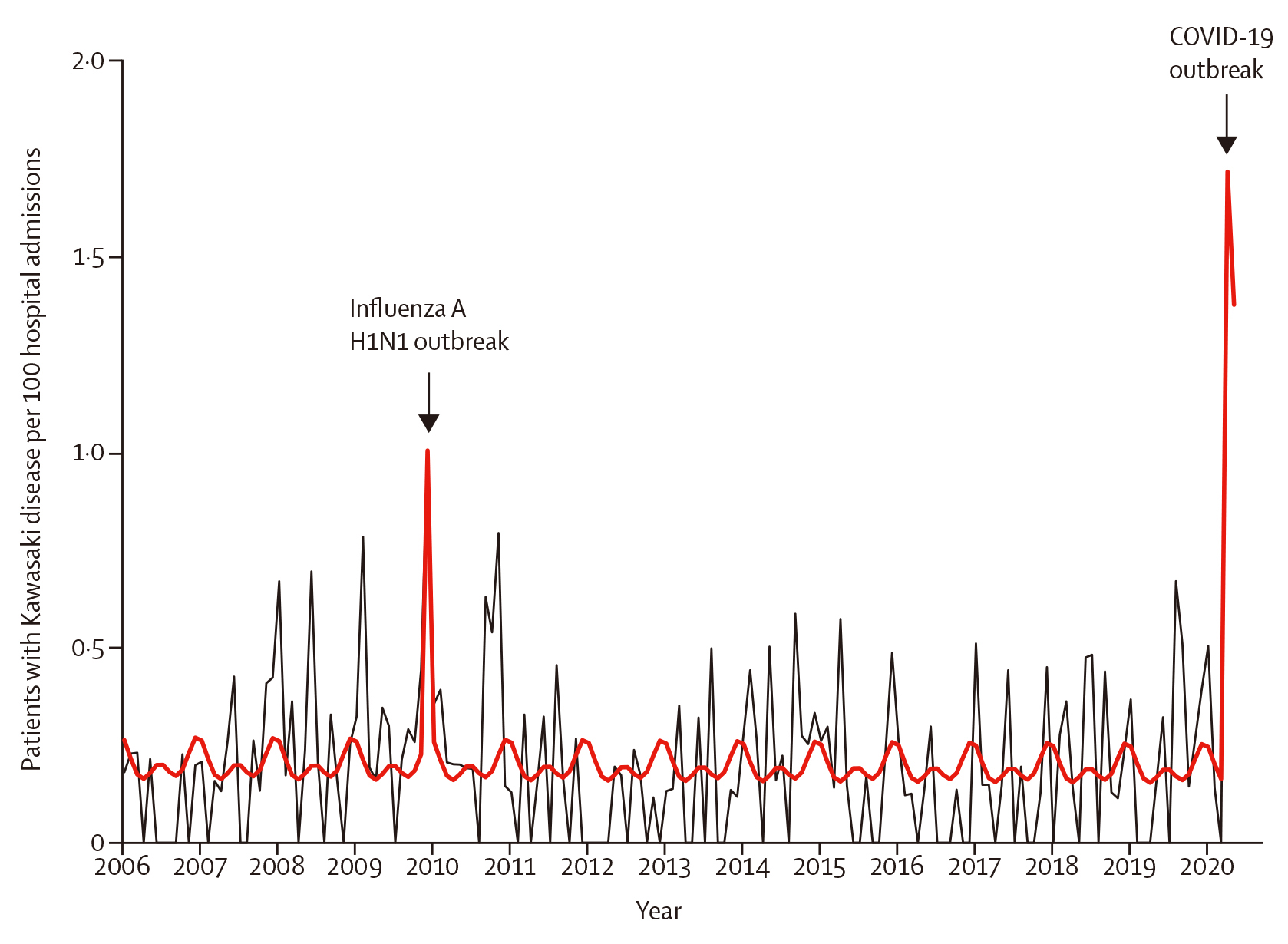 Figure 1. Kawasaki disease (KD) rate per 100 hospital admission, 2006 to 2020. 230 patients had KD during this period. The total number of hospital admission following paediatric emergency department visits was 110,824. The black line depicts the observed data. The bold red line depicts the model estimates based on the quasi-Poisson regression model. The influenza A H1N1 outbreak occurred between November to December 2009, and the COVID-19 outbreak occurred between March to April 20208.
Figure 1. Kawasaki disease (KD) rate per 100 hospital admission, 2006 to 2020. 230 patients had KD during this period. The total number of hospital admission following paediatric emergency department visits was 110,824. The black line depicts the observed data. The bold red line depicts the model estimates based on the quasi-Poisson regression model. The influenza A H1N1 outbreak occurred between November to December 2009, and the COVID-19 outbreak occurred between March to April 20208.
It is important to note that KD is often confused with SIRS (systemic inflammatory response syndrome) and this is one of the reasons for the delay in diagnosis9. The main clinical manifestations of both are fever, and laboratory tests include elevated white cell count (WCC), c-reactive protein (CRP), and procalcitonin. However, the treatment approach to these are completely different and therefore, an accurate diagnosis remains crucial9. Dr. Debono Sant Cassia explains that the main clinical features of KD are fever, bilateral non-exudative conjunctivitis, oral changes, erythematous rashes (typically appears during the acute phase of the disease and affects 80-90% of patients with KD) and, cervical lymphadenopathy. Those who do not meet the sufficient primary clinical presentation are diagnosed with incomplete KD and cervical lymphadenopathy is the main clinical manifestation in these subsets of KD9. Dr. Debono Sant Cassia suggested that since there are no specific biomarkers for KD, when dealing with children who exhibit prolonged fever for more than 7 days without a clear diagnosis, seeking consultation from a specialist is essential. Furthermore, the diagnostic process for KD, which relies on 5 key clinical symptoms, can introduce subjectivity, therefore, by involving a specialist, the likelihood of a correct diagnosis increases10.
Traditionally, patients with KD are treated with intravenous immunoglobulin (IVIG) to reduce the incidence of CAA from 5-25%. However, 10-20% of IVIG resistant patients (defined as having an oral or rectal temperature ≥ 38oC for at least 36 hours following the end of the initial IVIG infusion), there is no robust evidence to guide the treatment and this group of patients are at an increased risk of CAA11. Recent study data has suggested that the rate of IVIG resistance has increased from 7% in 2003 to 23% in 2014, with a concomitant increase in CAA12. Emerging studies on KD has explored other treatments which may perhaps reduce the number of cases related to treatment resistance seen in KD. In a multi-centred comparative effectiveness trial with patients aged between 4 weeks to 17 years who were diagnosed with IVIG resistant KD and having fever for at least 36 hours after completion of their first IVIG infusion were included in the study11. A total of 105 patients were randomly assigned treatment and 103 were included in the intention-to-treat population (54 in the infliximab group, 49 in the second IVIG group). The aim of the study was to compare infliximab with a second infusion of IVIG for treatment of resistance KD11.
Patients with fever 24 hours to 7 days following completion of first study treatment crossed over to receive the other study treatment. The primary outcome measure was resolution of fever at 24 hours after initiation of study treatment with no recurrence of fever attributed to KD within 7 days post-discharge11. Secondary outcome measured the duration of fever from enrolment, duration of hospitalisation after randomisation, and changes in inflammatory markers and coronary artery Z score11. The study found 77% of patients in the infliximab group and 51% of patients in the second IVIG infusion group met the primary outcome (odds ratio 0.31, 95% confidence interval [CI]: 0.13-0.73, p= 0.0076). Astonishingly, the mean hospital stay was 3.2 days (2.1) for the infliximab group and 4.5 days (2.5) for the second IVIG group (p <0.001). In addition, no patients who received infliximab experienced haemolytic anaemia compared to 15% who received IVIG either as first or second treatment11. The conclusion drawn from the study was that infliximab is safe, well tolerated, and effective for patients with IVIG resistance KD since it conferred to a shorter duration of fever, lower requirement for additional therapy, less severe anaemia and shorter hospitalisation compared with the second IVIG infusion11. Regarding the findings, Dr. Debono Sant Cassia highlighted the treatment unmet needs seen in patients resistant to KD and the utilisation of adjunctive therapies may help prevent further deterioration. Dr. Debono Sant Cassia¡¦s final remarks were that clinicians should remain vigilant and utilise the available tools to diagnose the condition as accurately as possible with the help of machine learning13.
References
1. Owens AM, et al. StatPearls Publishing LLC.; 2023. 2. Rife E, et al. Curr Rheumatol Rep 2020; 22(10): 75. 3. Wang H, et al. Lancet Child Adolesc Health 2023; 7(10): 697-707. 4. Elakabawi K, et al. Cardiol Res 2020; 11(1): 9-14. 5. James KE, et al. ACR Open Rheumatol 2021; 3(10): 671-83. 6. Mossberg M, et al. Rheumatology (Oxford) 2021; 60(4): 1910-4. 7. Li T, et al. J Int Med Res 2021; 49(3): 3000605211001712. 8. Ouldali N, et al. Lancet Child Adolesc Health 2020; 4(9): 662-8. 9. Li C, et al. Scientific Reports 2023; 13(1): 12553. 10. Kuo HC. et al. Int J Mol Sci 2023; 24(18). 11. Burns JC, et al. The Lancet Child & Adolescent Health 2021; 5(12): 852-61. 12. Kibata T, et al. Int J Cardiol 2016; 214: 209-15. 13. Portman MA, et al. Hosp Pediatr 2023; 13(3): 201-10.





