Discogenic low back pain (LBP) is a common clinical problem that more than 80% of adults would experience at some point in their lifetime. In addition to pain, discogenic LBP substantially worsens the functionality and quality of life of patients and incurs significant socioeconomic burdens to the society1. While discogenic pain arising from intervertebral disc (IVD) degeneration accounts for around 42% of all cases of non-specific LBP2, emerging investigations have been conducted for the development of therapies to maintain the normal physiological function and structure of the IVD , in addition to reverse its degeneration. Particularly, stem cell-based therapy (SCT), either as monotherapy or in combination with other therapeutics, appears to show some promising results..
Discogenic LBP is the most common type of LBP which is defined as to having one or more IVD identified as the root cause of the pain. Patients with the condition would have increased pain when sitting, coughing, or sneezing, and are relieved when standing or ambulation3. While IVD degeneration has been reported as the most prevalent contributor to LBP, the etiology of IVD degeneration is yet to be fully understood. Existing literature suggested that metabolic disorders, aging, mechanical loading, trauma, infection, nutrition, and genetic predisposition among the contributing factor for the development of LBP4.
Most IVD degeneration begins in early adulthood and progresses with age. The disappearance of notochordal cells in the nucleus pulposus and calcification of the cartilaginous vertebral endplates are considered to initiate and exacerbate the degenerative process. Given the roles of notochordal cells in generating proteoglycans and maintaining the gelatinous consistency, its loss in IVD will decrease proteoglycan synthesis in the tissue. Besides, calcification of the vertebral endplates decreases the supply of nutrients to the IVD, further exacerbating the degeneration process5.
Degenerated IVD is characterised by the reduced height and loading capacity associated with degraded extracellular matrix (ECM) and the loss of proteoglycans. As dehydrated nucleus pulposus is unable to balance the loads properly within the adjacent vertebrae, the loads eventually outspread the annulus fibrosus region causing mechanical and gradual structural destruction, resulting in herniation of the nucleus pulposus (Figure 1)5. As disc prolapse advances, together with nerve root compression or spinal stenosis, dermatomal radiation of pain to the lower extremity and neurological symptoms, such as numbness, motor weakness, and urinary or fecal incontinence, will be developed3.
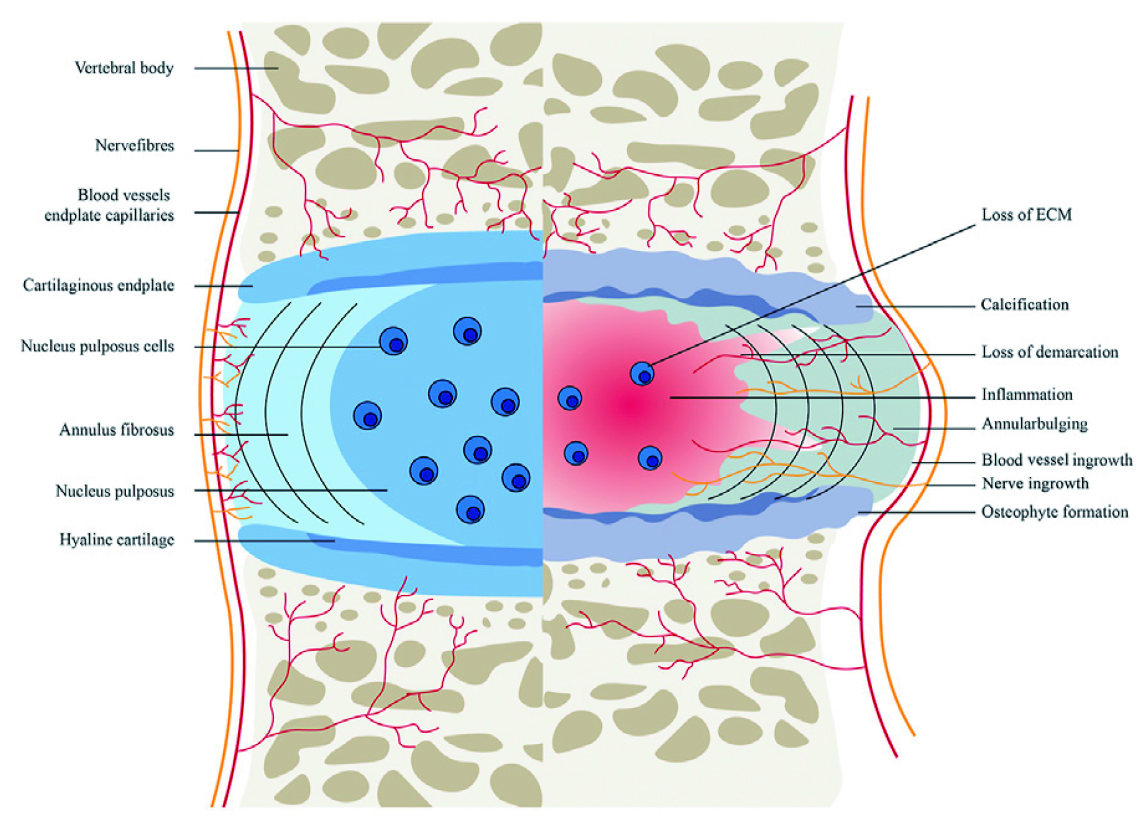
Figure 1. The healthy (left) and pathological (right) IVD4
There common strategies for IVD degeneration management include conservative and surgical treatments. For conservative treatment, the College of American Physicians’ clinical practice guidelines recommended non-pharmacologic treatments, such as exercise, acupuncture, mindfulness-based stress reduction, low-level laser therapy, cognitive behavioral therapy, and etc. In cases patients do not respond adequately to the non-pharmacologic treatments, non-steroidal anti-inflammatory drugs (NSAIDs) are the first-line treatment, whereas medications such as tramadol or duloxetine are recommended second-line therapy6. In advanced cases, corticosteroid injections can be given to treat radicular symptoms5. Notably, opioids should not be considered unless the patients have failed both therapies and the potential benefits outweigh the risks of opioid treatment6.
Surgical treatments, including interventional and conventional surgery, are normally limited to patients with severe or worsening symptoms after the conservative treatment has failed. Evaluation by diagnostic injection or provocative discogram is often required to assess the necessity of surgery5. Notably, both conservative and surgical treatments are mainly targeted to the alleviation of pain symptoms rather than the underlying pathogenesis or reversing the process of IVD degeneration3. Hence, a variety of innovative therapies targeting the IVD degeneration at the cellular and molecular levels and re-establishing IVD functions has emerged. SCT is a promising treatment strategy which attracted interest of researchers and clinicians.
Stem cells are multipotent that are able to self-renew and differentiate into specific cell types. In the context of countering IVD degeneration, stem cells can differentiate into IVD-like cells, particularly the nucleus pulposus cells. Furthermore, stem cells can support the viability of resident cells at the implantation site by secreting growth factors, chemokines, ECM components, and anti-inflammatory substances. Furthermore, stem cells are capable of slowing down the degenerative process by modulating immune function via the actions of anti-inflammatory cytokines and anti-metabolic mediators7. Remarkably, mesenchymal stem cells (MSC) therapy has been proposed to ameliorate discogenic LBP through three mechanisms: mitigate primary nociceptive disc pain, slow or reverse the catabolic metabolism, and restore disc tissue3.
Practically, adult stem cells, including hematopoietic and mesenchymal stem cells, can be found in most tissues throughout the body and are responsible for tissue maintenance2. In IVD tissue engineering, MSCs are collected from the bone marrow and amplified in-vitro. The cells are cultured further in the presence of the growth factors under hypoxic conditions, or in a three-dimensional environment, to produce the nucleus pulposus cell phenotype. The differentiated cells are seeded on a scaffold and injected into the damaged IVD (Figure 2)2. Essentially, given the pain and low yield of MSCs from bone marrow harvest procedures, the use of adipose tissue as an alternative source of autologous adult MSCs is currently being investigated due to their abundance and ease of harvest8.
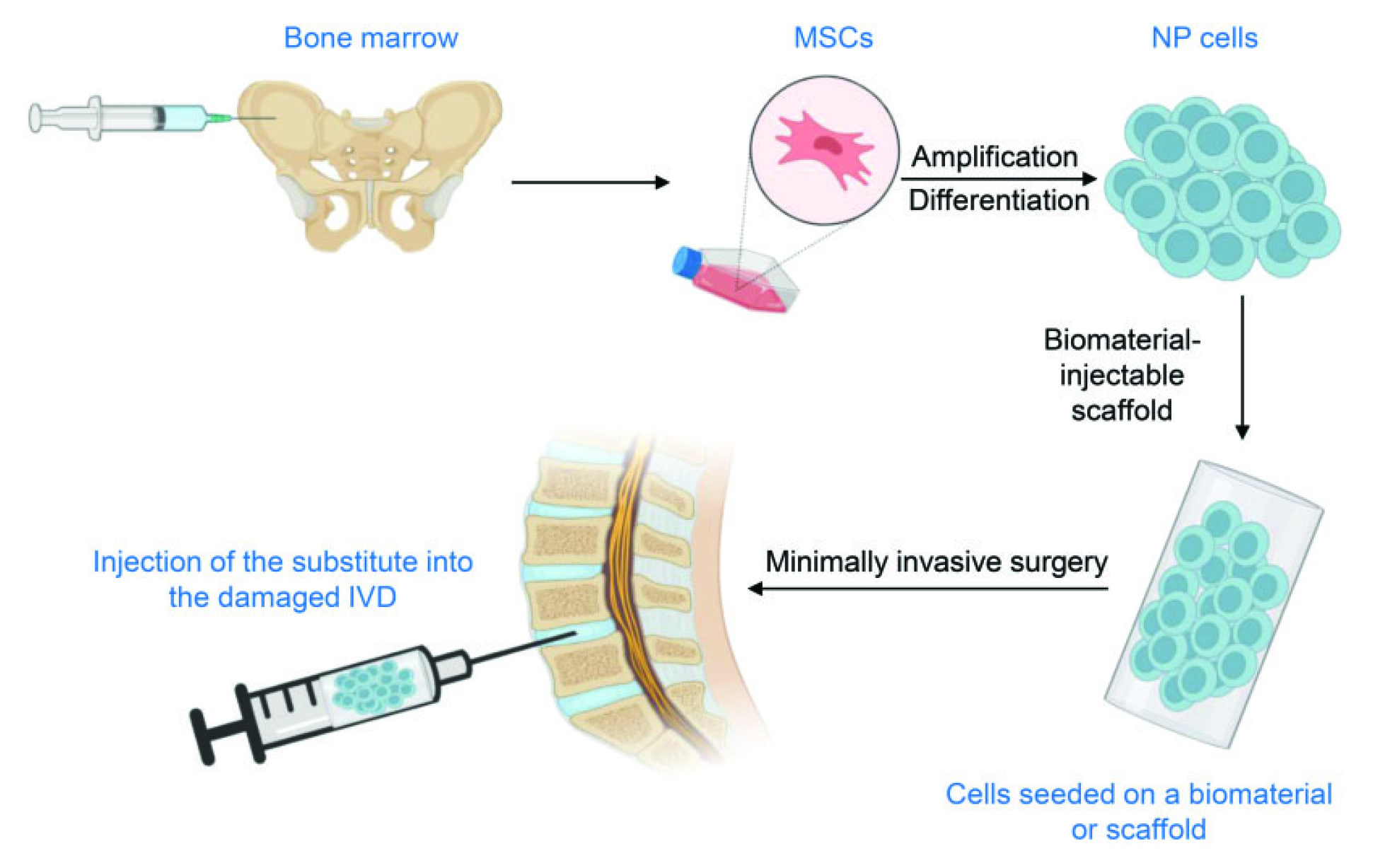
Figure 2. Procedures of SCT2, NP: nucleus pulposus
There are many studies that evaluted the efficacy and safety of SCT in treating IVD degeneration-associated LBP. For instance, in a recent 1-year feasibility study by Bates et al. (2022) involving 9 participants with chronic LBP originating from single-level lumbar disc disease, intradiscal injection of 10 million autologous adipose-derived MSCs with optional repetition after 6 months resulted in pain reduction in 7 (78%) of participants 12 months after treatment (Figure 3). 5 (56%) participants reported increased work capacity, whereas 3 (33%) participants reduced analgesic medication. Improvements in health-related quality of life, as reflected by EQ-5D, and Oswestry disability index results were observed. Of importance, MRI further demonstrated no further disc degeneration and improvements to annular fissures and disc protrusions. Moreover, there were no unexpected or serious adverse events recorded during the study8. Therefore, the results suggested that SCT is a safe and effective treatment option which can help control IVD degeneration and reduce discogenic LBP.
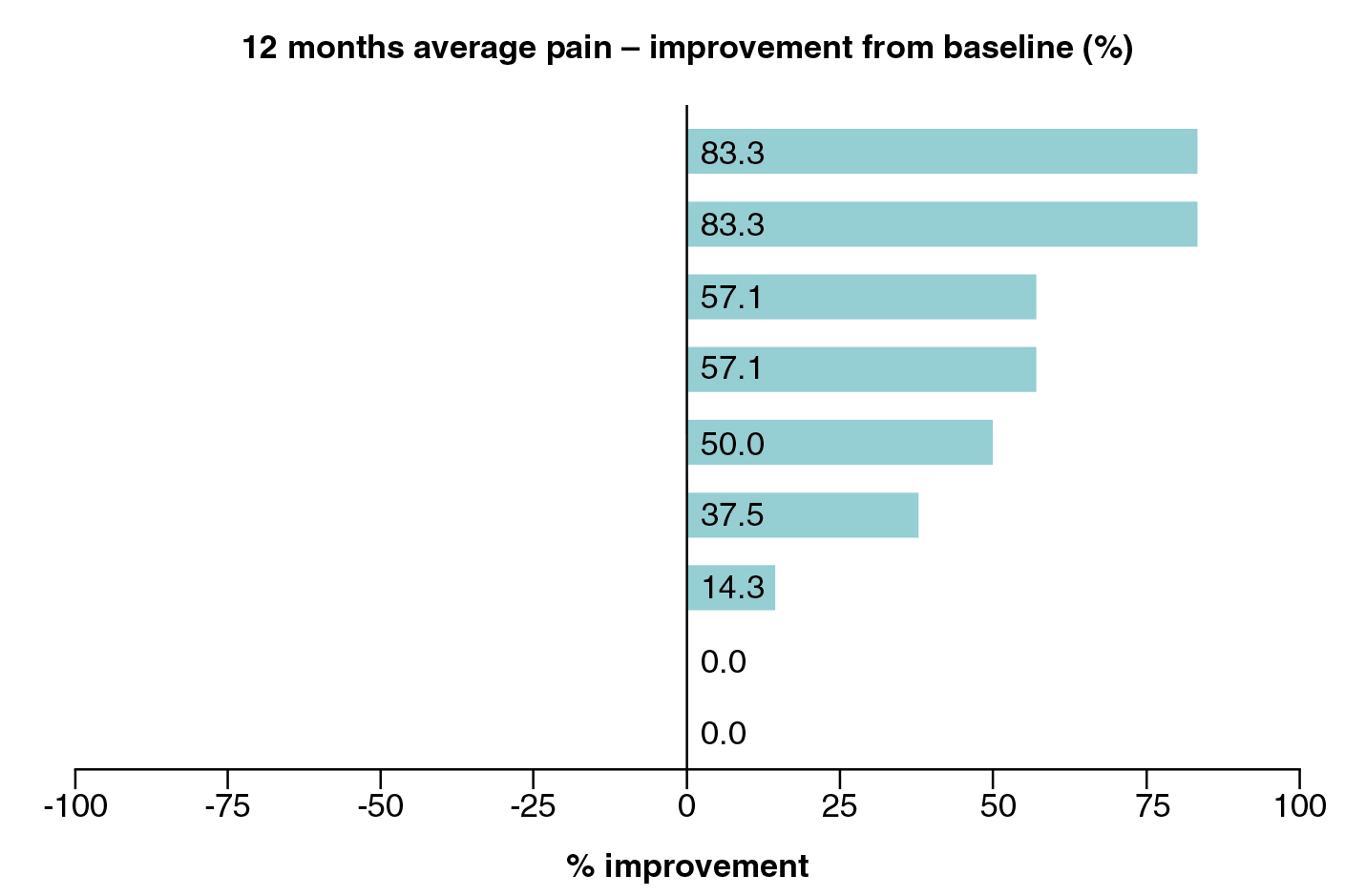
Figure 3. Patient-reported pain improvement 12 months after SCT8
Apart from applying as monotherapy, the therapeutic potential of SCT in combination with other therapies has also been evaluated. In the multi-centre randomised controlled trial by Amirdelfan et al. (2021), 100 subjects with chronic LBP associated with moderate IVD degeneration failing 3 months of conservative treatment were randomly allocated to receive 6 million MSCs with hyaluronic acid (HA), 18 million MSCs with HA, HA vehicle control, or saline control treatment. After 36 months follow-up, the MSC therapies was superior in improving LBP on a Visual Analog Scale (VAS) and Oswestry disability index compared with the controls. Additionally, the proportion of subjects achieving the minimally important change (MIC) and clinically significant change (CSC) composite endpoints for the MPC groups was also higher compared to the controls at various time points. The results also suggested that the MSC therapy was well-tolerated with no significant difference in rates of adverse events existed between MSC groups and controls9.
IVD regeneration following SCT shown some promising results since it was able revert degenerative changes and, provide a better control of LBP among sufferers. However, to maximise the reparative capacity and regenerative ability of SCTs, optimising the microenvironment in which stem cells are transplanted remains crucial in order to facilitate the long-term survival of the stem cells. For instance, hypoxia and thermal pre-conditioning have been reported to reduce the number of cells undergoing apoptosis and increase the potency through an unknown mechanism10. On the other hand, further development in the conditioned medium derived from growth factors, cytokines, chemokines, and hormones enhance the motility and migration capabilities of MSCs without affecting their multipotential ability. Thus, future investigations in the effects of the microenvironment in biological characteristics of MSC is strongly encouraged.
References:
1. DLiu et al. iScience 2022; 25: 104405. 2. Wang et al. J Int Med Res 2023; 51. DOI:10.1177/03000605231155777/ASSET/IMAGES/LARGE/10.1177_03000605231155777-FIG2.JPEG. 3. Urits et al. Curr Pain Headache Rep 2019; 23. DOI:10.1007/S11916-019-0804-Y. 4. Lu et al. Front Cell Dev Biol 2022; 9: 3966. 5. Romaniyanto et al. Ann Med Surg 2022; 77: 103619. 6. Kaye et al. Curr Pain Headache Rep 2022; 26: 751–65. 7. Zhang et al. Stem Cell Res Ther 2022; 13: 1–17. 8. Bates et al. Futur Sci OA 2022; 8. DOI:10.2144/FSOA-2021-0155. 9. Amirdelfan et al. Spine J 2021; 21: 212–30. 10. Chu et al. Front Bioeng Biotechnol 2022; 10: 1421.





