Chronic kidney disease (CKD) is one of the most prominent causes of death and suffering in the 21st century. The number of patients affected by CKD has substantially increased with an estimate of 843.6 million individuals globally in 20171. Even though the mortality related to end-stage kidney disease (ESKD) has declined, according to the Global Burden of Disease (GBD) studies, CKD is still emerging as a leading cause of mortality globally1. Moreover, the awareness of CKD is low since CKD is clinically silent and asymptomatic until the disease is in later stages. Notably, CKD is classified by the Kidney Disease Improving Global Outcome (KDIGO) into 5 stages. Stages 1 and 2 requires the presence of proteinuria, whereas stages 3-5 are defined by the changes in glomerular filtration rate (below 60 mL/min/1.73m2) over at least 3 months. Of note, CKD stage 3 is further divided into CKD stage 3a (estimated glomerular filtration rate [eGFR] 59-45 mL/min/1.73m2) and 3b (eGFR of 44-30 mL/min/1.73m2)2. The ongoing burden of CKD was also recently highlighted by the recent KDIGO updated on the CKD management 2023, along with the role of sodium-glucose cotransporter-2 inhibitors (SGLT-2is) in the management of CKD3.
CKD accounts for 1.43 million deaths globally in 2019 and as we know, a third of CKD patients are diabetic with cardiovascular disease (CVD). In fact, CKD is now ranked as the 11th leading cause of death, partially due to the ageing and an increasing burden of risk factors for CKD (diabetes and hypertension)3. Despite the increasing recognition of the true burden of CKD, screening for CKD or targeted screening programs for CKD remains controversial. Moreover, the disease awareness remains low with only 6% of general population and 10% of high-risk populations are aware of their CKD status, globally3. Furthermore, regardless of having a screening program in place, there are currently no perfect markers which can be used to provide a more accurate glomerular filtration rate (GFR). Traditionally estimation of glomerular filtration (eGFR) is based on plasma creatinine, nevertheless, since creatinine levels are affected by the muscle mass, and may be misleading at extreme body habitus or due to specific conditions (spinal cord injuries, sarcopenia)3.
A new biomarker for GFR, cystatin C, has been shown to be subjected to less biological interference and more sensitive to early declines in kidney function. In studies, cystatin C has also outperformed creatinine as an indicator of true GFR, in addition to add information regarding the occurrence of acute kidney injury4. The recent KDIGO updates on CKD management 2023 has recommended the use of both the cystatin C and creatinine since this combination provides a more accurate estimates of GFR stage when compared to measured values (Figure 1). However, it is also important to know that the cystatin C levels can be affected by different variables (steroid use, thyroid disease and cancer), therefore, may not be as reliable as a standalone marker to assess the change in GFR stages3.
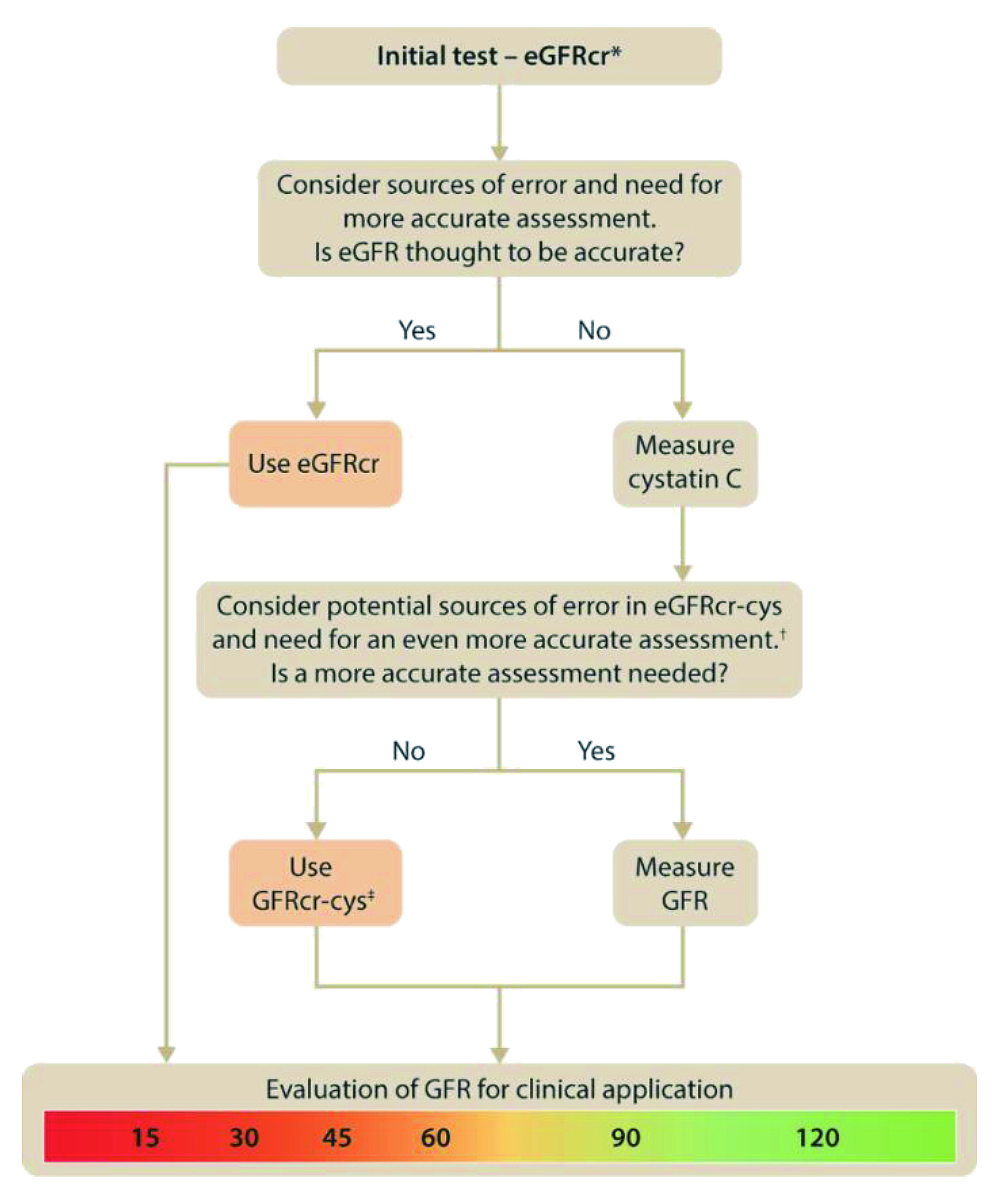
Figure 1. Approach to glomerular filtration rate (GFR) evaluation using initial and supportive tests. KDIGO suggested to initially use supportive testing to develop a final assessment of true GFR and to apply it in individual decision making. The initial test for evaluation of evaluation of GFR is creatinine-base estimation GFR (eGFRcr) and if eGFRcr is expected to be inaccurate or if a more accurate assessment of GFR is required such a diagnosing or staging of CKD or drug dosing, then cystatin C should be measured, and creatinine and cystatin C-based estimated eGFR (eGFRcr-cys) should be estimated. *Initial test may be estimated GFR by cystatin C (eGFRcys or eGFRcr-cys) in otherwise healthy populations with changes in creatinine generation due to non-GFR determinants such as changes in muscle mass or creatinine secretion or extrarenal elimination due to use of specific medications. †Sources of error in eGFRcr-cys include very low muscle mass or very high levels of inflammation, high catabolic states, exogenous steroid use. ‡Consider eGFRcys rather than eGFRcr-cys in otherwise healthy populations with decreased creatinine generation due to reduced muscle mass or decreased creatinine secretion or extrarenal elimination due to use of specific medications3.
Diabetes is the leading cause of CKD globally, accounting for nearly half of all cases of kidney failure requiring renal replacement therapy. In addition, patients with type 2 diabetes (T2D) and CKD are more likely to die early from atherosclerotic cardiovascular disease (ASCVD) and heart failure (HF)5. Very recently, the SGLT-2is such as dapagliflozin which has been used for the treatment of T2D has shown to reduce the risk of CV death and hospitalisation for heart failure (HHF). In addition, the renal benefits of dapagliflozin were also investigated by Heerspink et al., in the DAPA-CKD clinical trial in 2020. The trial included 4,304 participants with an eGFR of 25-75 ml/min/1.73m2 and urinary-albumin-to-creatinine of 200 to 5000 randomly (with albumin measured in milligrams and creatinine measured in grams) assigned to receive dapagliflozin 10 mg once daily or placebo. The primary outcome was a composite of a sustained decline in the eGFR of at least 50%, end-stage kidney disease, or death from renal or CV causes. The study concluded that dapagliflozin reduced the risk of eGFR decline by at least 50% with 44% reduction in death from the renal cause (Figure 2)6. In addition, CV death, or HHF was 29% lower in cohorts on dapagliflozin compared to placebo6.
To further substantiate the DAPA-CKD trial findings, a post hoc trial analysis on patients with CKD, and their risk of CVD, as well as renal events were assessed. The analysis concluded that dapagliflozin reduces the risk of kidney failure, death from CV causes or HHF, and prolonged survival in individuals with CKD, regardless of their diabetic status, independent of the presence of concomitant CVD7. Furthermore, acute hospitalisations are common in patients with CKD, and often leads to a decrease in health-related quality of life in these patients. A post hoc analysis of DAPA-CKD performed by Schechter et al., 2022 assessed the effects of dapagliflozin on first hospitalisations and all (first and subsequent) hospitalisations related to CKD. The study included 4,304 patients with a mean age of 61.8 years and 33.1% females with a median follow-up of 2.4 years. The study concluded that dapagliflozin reduced the risk for a first hospitalisation (hazard ratio, 0.85, 95% Cl: 0.75-0.94) and all hospitalisations or death. These reductions were again independent of the diabetic status8. The beneficial effects dapagliflozin reported by the DAPA-CKD trials were also highlighted in the KDIGO 2022 Clinical Practise Guideline for Diabetes Management and it recommended treating patients with T2D, and CKD with an eGFR ≥ 20 mL/min/1.732 with an SGLT-2is (1A recommendation)9. The very recent KDIGO updated in 2023 has further recommended to continue SGLT-2i even if the eGFR falls below 20 mL/min/1.73m2, unless it is not tolerated, or kidney replacement therapy is initiated. Notably, the recent guideline updates of KDIGO advocated SGLT-2i use in adults with CKD and HF or eGFR ≥ 20 mL/min/1.73m2 with urine albumin-to-creatinine ratio (ACR) make sure this is on the same line (2B recommendation)3. Apart from the KDIGO recommendations, the updates of the 2023 European Society of Cardiology (ESC) guidelines has also recommended SGLT2is such as dapagliflozin for patients with T2D and CKD since randomised clinical trials has shown it to reduces the risk of HHF or CV death especially in patients with T2D and CKD (Class 1A recommendation)10. In conclusion, addition of dapagliflozin to standard therapeutic management of CKD provides long-term cardiorenal benefit and may help lower the burden of CKD in vulnerable populations.
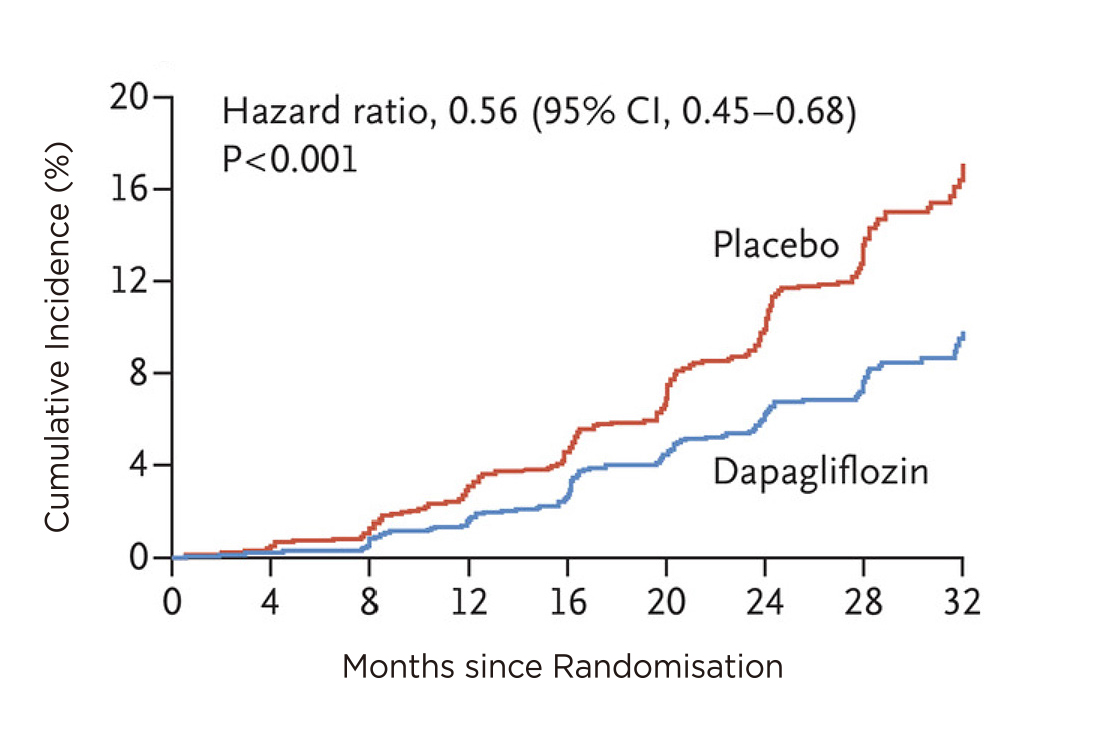
Figure 2. Primary composite outcome of a sustained decline in the eGFR of at least 50%, end-stage kidney disease, or death for renal cause. Dapagliflozin showed a sustained reduction of the primary composite outcome compared to placebo6.
References:
1. Kovesdy CP. Kidney Int Suppl (2011) 2022; 12(1): 7-11. 2. Kampmann JD, et al. BMC Nephrology 2023; 24(1): 17. 3. KDIGO. Kidney Int 2023. 4. Kar S, et al. J Clin Pharmacol 2018; 58(10): 1239-47. 5. Tuttle KR, et al. Diabetes 2021; 70(1): 1-16. 6. Heerspink HJL, et al. New England Journal of Medicine 2020; 383(15): 1436-46. 7. McMurray JJV, et al. Circulation 2021; 143(5): 438-48. 8. Schechter M, et al. Ann Intern Med 2023; 176(1): 59-66. 9. KDIGO. Kidney Int 2022; 102(5s): S1-s127. 10. McDonagh TA, et al. European Heart Journal 2023.