
Managing Diabetic Peripheral Neuropathy with Topical Therapeutics
Neuropathic pain is a disorder of the central and peripheral nervous system resulting from a lesion or disease. In diabetic patients, neuropathic pain (referred to as diabetic polyneuropathy, DPN) is one of the most common complications whereas painful diabetic peripheral neuropathy (PDPN) is a common form of DPN1, with a prevalence of 5.8% to 34% in type 1, type 2, or overall diabetes mellitus (DM) patients2. Unfortunately, there is no single medication for preventing or reversing neuropathic changes, or providing total pain relief3. However, there is increasing evidence on the analgesic efficacy of topical therapeutics for DPN. With the reported adequate safety and continuous long-term treatment efficacy, topical therapeutics would be ideal options of complementary therapies for DPN.
The Unmet Need in Management of DPN
The management of diabetic polyneuropathy (DPN) is challenging and a multi-modal approach, involving early diagnosis, glycaemic control, psychological therapy and symptomatic pain relief, is often required4. Currently, both oral and topical agents are available for treating DPN whereas oral medications are used by the majority of patients suffering from neuropathic pain. The main problem associated with oral medications are the lack of efficacy in a large proportion of patients that a former report suggested only one-third of patients achieved satisfying pain relief5. Besides, the occurrence of side effects, including xerostomia, dizziness and nausea, and potential drug-drug interactions hamper acceptance towards oral therapies as well. Pharmacologically, the onset of treatment effects of oral analgesic drugs is so slow that an intake period of 6–8 weeks at the maximum dose is needed for certain drugs before the efficacy can be judged. For topical agents, although less hazardous in general, practical inconvenience such as potential contamination of hands or clothes during application is a reported disadvantage6.
Pathogenesis of DPN
Although the exact mechanism involved in the generation of DPN is not completely understood, a variety of potential factors eliciting the pain have been postulated. In particular, it is generally accepted that hyperglycaemia plays a crucial role in the development of DPN, likely by decreasing the pain thresholds7. Notably, the increase in advanced glycation end-products (AGEs) production and decrease in regeneration of glutathione may be caused by hyperglycaemia8. Depletion of glutathione would lead to oxidative stress and accumulation of toxic species. Besides, hyperglycaemia would also trigger nerve hypoxia, especially in sensory nerves, leading to alteration in nerve functions and electrical stability9. AGEs, oxidative stress and nerve hypoxia cause the production of inflammatory cytokines and growth factors, which in turn, trigger neuroinflammation and nerve injury10,11. In addition, the upregulation of transient receptor potential vanilloid 1 (TRPV1) expression has been identified to be associated with neuropathic pain12. The nerve injury caused would result in abnormal ectopic discharges and abnormal synaptic neurotransmitter release, hence triggering development of neuropathic pain (Figure 1)13.
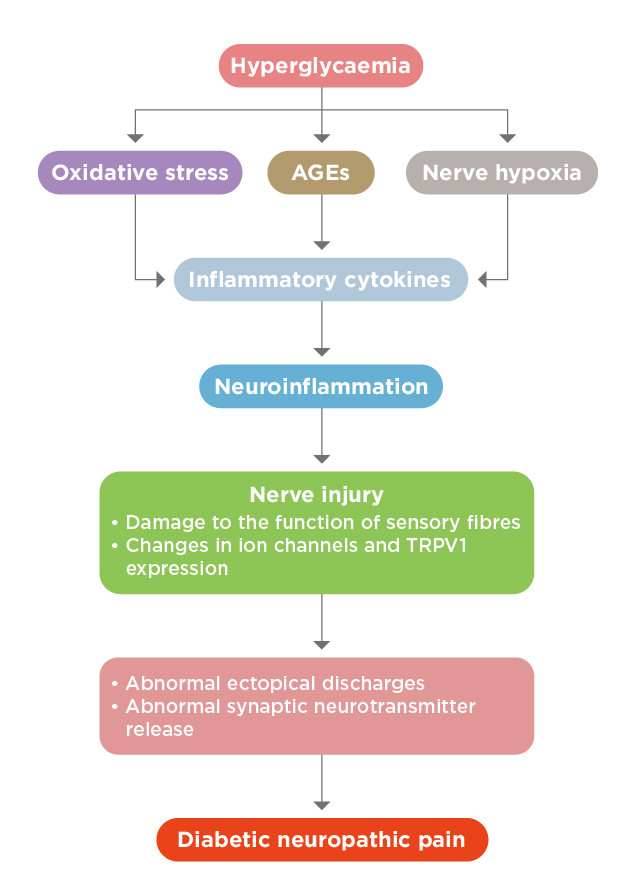
Figure 1. The pathogenesis of DPN13
Topical Therapeutics for DPN
There are various types of topical therapeutics developed for treating DPN and the therapeutics act on different targets in the cascade of pain generation. For instance, lidocaine, a blocker of voltage-gated sodium channels, stabilises the neuronal membrane potential on abnormally excitable Aδ and C fibres leading to a reduction of ectopic discharges14. Besides, capsaicin, a natural occurring alkaloid in chili peppers, binds to TRPV1 expressed on Aδ and C fibres, causing the opening of TRPV1 channels. This leads to sodium and calcium influx and release of substance P. Repeated TRPV1 exposure to capsaicin causes substance P depletion and TRPV1 desensitisation and defunctionalisation15. On the other hand, amitriptyline, a tricyclic anti-depressant, acts by blocking Na+, K+, and Ca2+ voltage-gated ion channels and thus inhibiting neuronal reuptake of norepinephrine and serotonin16.
The treatment efficacy of topical therapeutics in controlling DPN has been demonstrated in clinical trials. In a double-blind, randomised and non-inferiority trial, 102 patients with type 2 diabetes and chronic daily pain for more than 3 months were randomly allocated to receive either amitriptyline 2% cream or capsaicin 0.75% cream 3 times a day for 12 weeks. The results showed that both treatments yielded significant pain relief in 12 weeks as compared to the baseline values (p<0.001 for both). Nonetheless, intention-to-treat analysis showed no difference in the efficacy between the 2 treatments (p=0.703, Figure 2). However, adverse events were more common in the capsaicin group (p=0.001), whereas major side effects were itching, blister formation and erythema17.
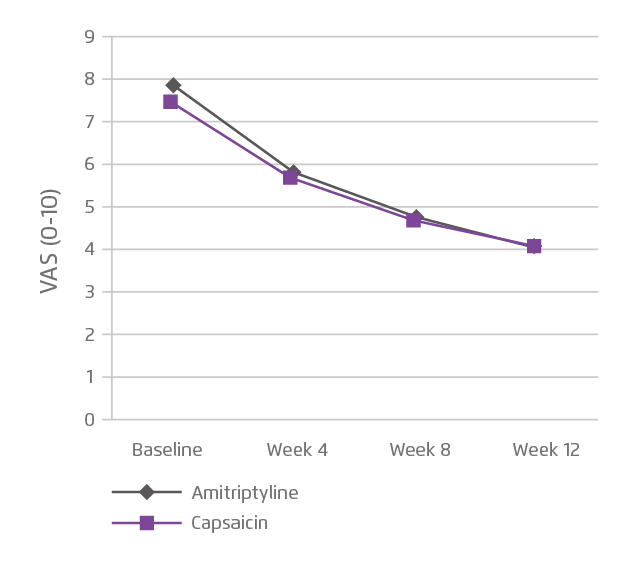
Figure 2. Efficacy of topical amitriptyline and capsaicin on pain management17
On the other hand, a recent meta-analysis including 25 randomised controlled trials showed that topical capsaicin was as effective as oral agents in managing PDPN and was more tolerable. In this analysis, topical capsaicin patch was significantly more effective than placebo in pain reduction (odds ratio: 2.28, 95% CI: 1.19-4.03) and had similar efficacy as compared to oral agents (pregabalin, duloxetine and gabapentin) in patients with PDPN. Also, the oral agents were associated with a significantly elevated risk of side effects including somnolence, dizziness, fatigue, and discontinuation due to adverse events compared with placebo18.
Optimisation of DPN Control
The management of DPN is still a challenging clinical condition as its pathophysiology is not yet fully understood and pain relief is unsatisfactory. Nonetheless, a wide variety of therapeutics have been developed in recent years and their efficacy in controlling pain has been demonstrated in clinical trials. In particular, topical therapies were shown to have better safety profiles as compared to oral agents without compromising efficacy. However, further investigations into the combined effects of different therapies and, importantly, research on the pathophysiology of DPN are vital for optimising the clinical outcomes of DPN treatments.
References
1. van Nooten et al. Pain Res Treat. 2017;2017:6080648. 2. Alleman et al. Diabetes Res Clin Pract. 2015;109(2):215-225. 3. Javed et al. Ther Adv Chronic Dis. 2015;6(1):15-28. 4. Kaku et al. Curr Diab Rep. 2015;15(6):35.5. Jensen et al. Curr Opin Neurol. 2009;22(5):467-474. 6. Üçeyler et al. Pain Ther. 2014;3(2):73-84. 7. Morley et al. Am J Med. 1984;77(1):79-82. 8. Babizhayev et al. Cell Biochem Biophys. 2015;71(3):1425-1443. 9. Fuchs et al. Pain. 2010;151(2):496-505. 10. Tóbon-Velasco et al. CNS Neurol Disord Drug Targets. 2014;13(9):1615-1626. 11. Gao et al. Eur J Pharmacol. 2017;814:144-150. 12. Aslam et al. Pain Res Treat. 2014;2014:1-7. 13. Yang et al. Exp Ther Med. 2019;17(3):1963-1976. 14. Krumova et al. Pain. 2012;153(2):273-280. 15. Schreiber et al. World J Diabetes. 2015;6(3):432-444. 16. Watson. Clin J Pain. 2000;16(2 Suppl):S49-55. 17. Kiani et al. Iran J Pharm Res IJPR. 2015;14(4):1263-1268. 18. van Nooten et al. Clin Ther. 2017;39(4):787-803.e18.





