Aging is a process of chronic, normal culmination of the loss of specific regenerative and bioprotective mechanism that occurs over a period. In addition, at cellular level, primary aging-related mechanisms occurs as cell proliferation slows to a point where there is a total cessation eventually. As we age through young and middle adulthood, the overall amount of these senescent cells within our bodies remains relatively low and manageable to overcome the body’s still higher number of cells, which are not yet senescent and functioning in line with normal physiology1. As we know that the world’s population is ageing rapidly, with a significant change in population demography reported in high to low income countries2. In fact, it is estimated that the number of adults above the age of 65 will reach around 1.5 billion by the year 2050, and this will undoubtedly put significant strain on the healthcare services globally1,3. So, what are the hallmarks of ageing? Since aging is characterised by a progressive loss of physiological integrity, leading to an impaired function, and increasing prevalence of major human diseases such as cancers, diabetes, cardiovascular disorder, and neurodegenerative diseases. Furthermore, genomic instability, telomere attrition, epigenetic alterations, stem cell exhaustion are all hallmark changes seen related to the aging process4. Nonetheless, the quest for longevity has driven the anti-aging research to the next level and the development of potential drugs for aging-related diseases5. Very recently, animal studies have shown transfusion of blood components from young to aged animals improved their cognitive functions and suggested a role of rejuvenation of aging body organs.
Virtually all organ systems are involved in physiologic changes associated with aging and it is essential to distinguish the normal processes of aging from the pathologic changes that occurs in the setting of a disease but are markedly more drastic due to the decreased or total loss of compensatory mechanisms. For instance, abnormal compensatory mechanisms due to aging increases the risk development of neurodegenerative disorders in older adults1. But why? The reason behind this is related to the fact that human cells have finite number of times that they can replicate and divide before they become senescent. Moreover, research has also shown that telomeres on the DNA strand gradually shortens as a cell divides. Therefore, the protective qualities are lost overtime exposing the ends of the chromosomes allowing DNA repair enzymes to recognise telomeres amongst sites of DNA damage. This then activates the tumour protein, p53 and other cascade cyclin-dependent kinase inhibitors which subsequently causes senescence of cells; ultimately, this leads to the cessation of their metabolic and replicative functions1.
In relation to the aging population crisis, the prevalence of sarcopenic obesity (SO) is rising and concerning, especially in older adults with a prevalence of 20% globally. So, what is the relationship between SO and aging? Well, SO is a complex condition characterised by a dual burden from sarcopenia and excess fat, which inadvertently predisposes older adults to frailty, falls, disability, immobility fractures and cancers6. This in itself is an important public health concern since SO is associated with increased morbidity and mortality among older adults, in addition places a heavy burden on individuals, society and the healthcare system6. It is only one of the age-related pathologies among many, therefore, urgent interventions are needed to improve the health and longevity among this vulnerable population. Nevertheless, it is natural to question whether aging can be partially reversed or at least improved to reduce health related decline. The answer to this is somewhat complex, but it is worth exploring the current research findings into this area which we shall highlight. The idea of rejuvenating body clock through transfusion of young blood was initially reported over 15 years ago in animal studies. It showed older rodents receiving transfusion from younger rodents had a degree of regeneration of brain, muscles, and other tissues in a process called parabiosis. Recently, scientists performed parabiosis procedures in animal models where the circulatory system of young and old mice were sutured together to assess the tissue regeneration ability in the older mice (Figure 1). To their surprise, they noted an increase in the growth of neurons in the hippocampi of older mice related ten-eleven translocation methylcytosine dioxygenase 2 (Tet2), thereby, improving their cognitive performances. Nonetheless, when this enzyme was inhibited, the cognitive function declined and when it was overexpressed, the cognitive function improved to levels similar to that of the younger mice7. However, such procedures are not feasible in reality since they carry a high risk of infection, antibody activations, in addition to having a high mortality rate secondary to parabiotic disease which is the common cause of death in animal subjects exposed this process8.
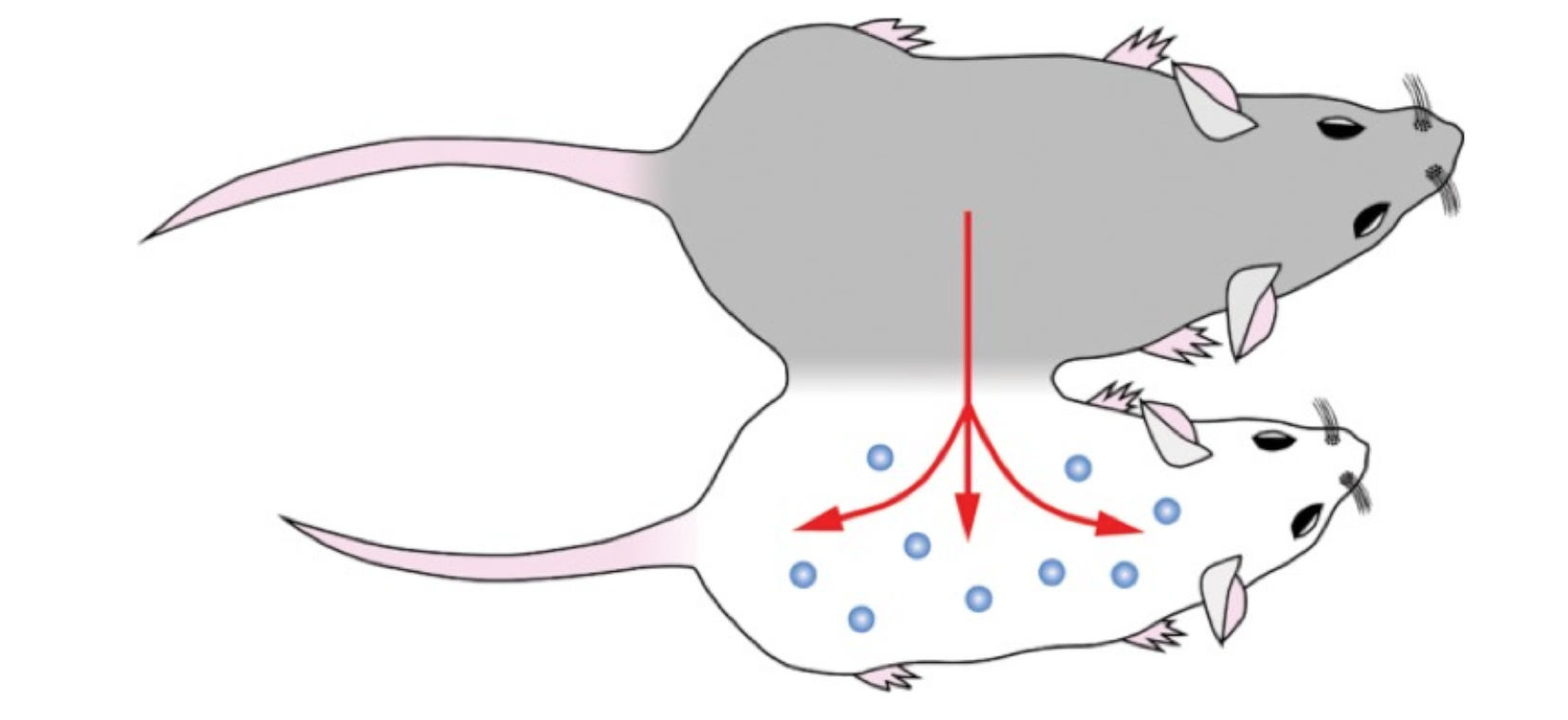
Figure 1. Parabiosis experiment that connected the circulatory systems of young and older mice7
Now some may ask whether the young blood being the holy grail for anti-aging? The answer to this is no, since it does not reverse the aging process, however, research into this area can help scientists to understand more on the regenerative factors which can be used to treat diseases. Furthermore, the research to slow or halt aging is complex since parabiosis as discussed earlier not only requires the two organisms to share their circulatory system, instead they also share their immune and organ systems, thereby making it difficult to rule out the influence of other system on aging or rejuvenation. Notably, parabiosis-like techniques have been explored further and researchers were able to exchange only blood between the young and older mice. However, the results were not as promising since it had a negative effect on the young mice as the old blood transfused from older mice resulted in a decrease in hippocampal neuron generation, learning and agility in young mice. Contrary to this, young blood transfused to older mice showed no significant benefit on cognition, agility, or generation of hippocampal neurons in older mice. The conclusion drawn from the study was that the secret to stall aging may not lie in boosting rejuvenating factors, instead to block factors in old blood which may induce or promote the aging process7.
Then what about the stem cell transplant, can they reduce the burden of aging? Very recently, a study performed by Shamagian et al., (2023), suggested transplanting young cardiosphere-derived cells (CDCs) showed some anti-aging effects of heart function in older rodents. They found that the extracellular vesicles (EVs) from CDCs (CDC-EVs) were able to mimic the anti-senescent effects of CDCs through activation of telomerase-telomere axis. In fact, the telomere length was twice as long in CDC-EV treated animals compared to the control. Physiologically, this translated to a regression of LV hypertrophy, weight and brain natriuretic peptide (BNP) levels in CDC-EV treated animals (14.5% decrease in LV mass at
1 month, p= 0.001) compared to the control (Figure 2)3. The beneficial effects were maintained during the early follow-up period with changes observed in multiple organs during assessment. The study concluded that neonatal CDC-EVs in old animals, and in-vitro on senescent human cells induced multiple broad anti-aging effects3. Despite these positive findings, the results obtained from rodent model does not recapitulate the aging process in human and the safety regarding stem cell use remains a major question3,9.
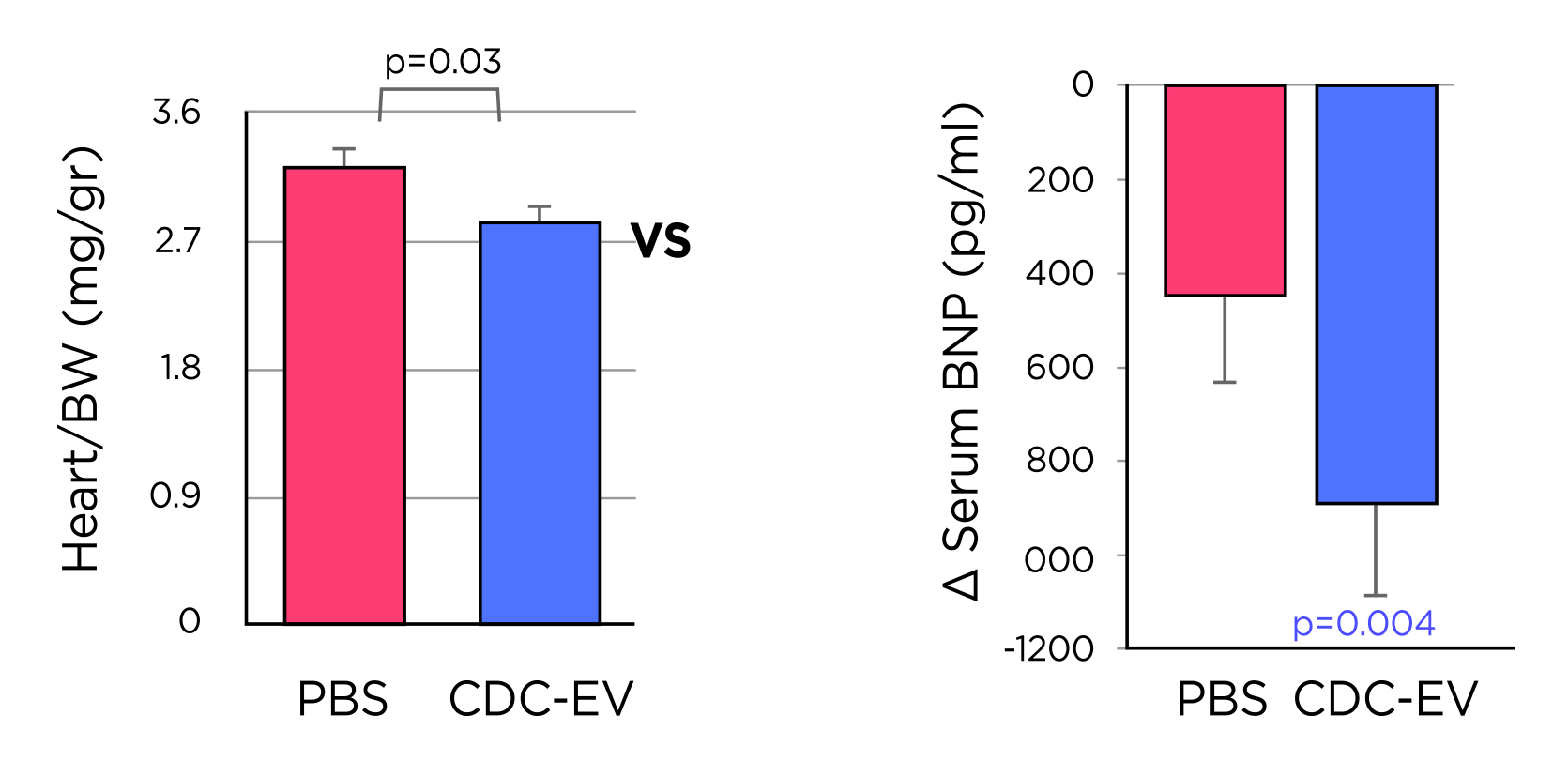
Figure 2. The effects of CDC-EV shown: (a) heart/body weight ratio (BW) being lower in CDC-EV (n=6) vs PBS (n=7) treated rodents, (b) The decrease in serum levels of BNP was significant after 16 weeks of treatment in CDC-EV group (n=7) while in the control PBS rodents (n=7), the changes was not significant3. BNP= brain natriuretic peptide; BW= body weight; CDC-EV= cardiosphere-derived cells-extracellular vesicle; PBS= phosphate buffered saline.
Even though the field of rejuvenation research is growing, the underlying components in the blood of young animals responsible for reversing age-related changes remains elusive. Nonetheless, reports from heterochronic parabiosis led studies in which systemic administration of blood plasma preparation derived from young or exercised mice helped rejuvenate the aged brain. These plasma preparations were in largely devoid of cellular components, therefore, a study performed by Schroer et al., 2023 investigated the cellular and molecular mechanisms in the blood of young animals that drove the beneficial effects seen during young blood plasma administration in aged animals10. Aged male mice were injected with either young blood plasma preparation, the young platelet fraction or saline (100µL per injection) 8 times over 24 hours10. The study found that platelet factors transferred the restorative effects of young blood on immune and cognitive function to the aged brain. In addition, authors highlighted how the platelet derived chemokine platelet factor 4 (PF4) released as exerkines by the platelets may have attenuated age-related neuroinflammation and rescued the hippocampal-dependent learning and memory in aged mice10.
Moreover, the PF4 was believed to exhibit its function by utilising chemokine receptor CXCR3, which may have attenuated PF4 effects on cellular, molecular, and cognitive functions in aged brain. Notably, the age-related cellular changes in myeloid cells, T-cells and natural killer cells are implicated as the drivers of decreased regenerative capacity, increased senescence, and cognitive impairments in the ageing brain. Given the strong associated between the increased inflammation and age-related neurodegeneration diseases such as Alzheimer’s disease, the beneficial effects of platelet factors may extend more broadly to dementia-related disorders in older adults10. The effects of platelets induced regeneration has also been reported in osteoarthritis as platelet-rich plasma (PRP) from young individuals induced a regenerative effect in aged cells and mice when injected, whereas PRP from aged individuals showed no improvement in chondrocyte health or cartilage integrity11. Regardless of the promising results demonstrated in these studies, they may not be replicable in human subjects and the beneficial effect may not be applicable to all individuals equally since the process of aging remains complex and driven by various factors. In conclusion, transfusion of PRP showed some promising results, however, the availability of these may not equally be available to everyone, especially those in need. Therefore, more long-term randomised controlled trials in human subjects are required to assess the anti-aging effects of different compounds in the human population prior to considering their therapeutic role in diseases related to aging.
References:
1. Flint B, et al. StatPearls Publishing, 2023. 2. Mitchell E, et al. British Journal of Hospital Medicine 2020; 81(2): 1-9. 3. Grigorian Shamagian L, et al. Scientific Reports 2023; 13(1): 12240. 4. López-Otín C, et al. Cell 2013; 153(6): 1194-217. 5. Li Z, et al. Biogerontology 2021; 22(2): 165-87. 6. Ji T, et al. Aging Dis 2022; 13(2): 379-88. 7. Pandika M. ACS Cent Sci 2019; 5(9): 1481-4. 8. Conese M, et al. Open Med (Wars) 2017; 12: 376-83. 9. Bajaj J, et al. J Cell Biol 2020; 219(1). 10. Schroer AB, et al. Nature 2023. 11. Chowdhary K, et al. Am J Phys Med Rehabil 2023; 102(7): 597-604.





