Tendinopathy is a common chronic degenerative musculoskeletal condition affecting various body parts, including the Achilles, patellar, rotator cuff, and forearm extensor tendons. The incidence of tendinopathy continues to rise due to an increase involvement of humans in recreational sports1. While the exact pathogenesis of tendinopathy is yet to be fully understood, recent investigations suggested that neuronal regulation by various neuropeptides plays an essential role in tendon homeostasis, and the disruption of this regulation contributes to the development of neurogenic inflammation and tendinopathy2. In particular, substance P (SP) has been reported as the mediator of inflammation and tendinopathy3. In contrast, some studies revealed that SP being an essential component of tendon healing4. The current review aims to discuss the roles of SP in the development of tendinopathy and evaluate its potential as a therapeutic target for countering the disorder.
Achilles tendon (AT) is the site most susceptible to tendinopathy, especially in athletes due to stress induced by repetitive impact activities, such as running and jumping. For instance, 42% of middle and long-distance runners experience Achilles tendinopathy before age 455. Additionally, AT is also prevalent in middle-aged, overweight individuals and those physically inactive6. This disease contributes to pain, disability, and early retirement from sports and work. Apart from physical outcomes, tendinopathy may induce mental distress and often increase medical expenditure7.
Tendon homeostasis is governed by the tight balance in time, space, and quantity of resorption and formation processes. Mechanical loading is a well-known extrinsic factor affecting tendon protein synthesis and degradation. Notably, a reduction in mechanical stress has shown to reduce tendon elastic properties in both humans and animals. In vivo studies including younger and older men by Couppé et al. (2012) demonstrated a decrease in tendon stiffness and Young’s modulus upon 14 days of immobilisation8. On the contrary, upon mechanical loading, a loss of collagen occurs initially, but a net gain in collagen is achieved over time. Nonetheless, when the repeated loading exceeds the tendon’s capacity to form new collagen, tendinopathy is induced2.
Tendon homeostasis is governed by the tight balance in time, space, and quantity of resorption and formation processes. Mechanical loading is a well-known extrinsic factor affecting tendon protein synthesis and degradation. Notably, a reduction in mechanical stress has shown to reduce tendon elastic properties in both humans and animals. In vivo studies including younger and older men by Couppé et al. (2012) demonstrated a decrease in tendon stiffness and Young’s modulus upon 14 days of immobilisation8. On the contrary, upon mechanical loading, a loss of collagen occurs initially, but a net gain in collagen is achieved over time. Nonetheless, when the repeated loading exceeds the tendon’s capacity to form new collagen, tendinopathy is induced2.
Emerging data suggest that the nervous system plays an active role in regulating pain, inflammation, and tendon homeostasis through specific neuronal mediators. Remarkably, histological investigations revealed that new ingrowth of sensory nerve fibres are present in tendons with tendinopathy (Figure 1), which is also observed during tissue proliferation in healing tendons2. In this regard, neurogenic inflammation arising from the local release of neuronal mediators would explain the development of tendinopathy symptoms, such as pain and oedema, despite the lack of infiltration of inflammatory cells1.
The neuronal dysregulation in tendinopathy is characterised by an aberrant increase in sprouting sensory nerves and the increased expression of SP2. SP is a neuropeptide widely distributed in both the central and peripheral nervous systems. Non-neural cells, such as endothelial cells, and bone marrow stromal cells, are also shown to produce SP9. While SP is reported to be associated with mediating pain, oedema, and fibrosis in tendinopathy, research findings indicates that SP is an essential component in the healing of acute tendon injury or rupture through multiple functions, such as increasing fibroblast proliferation and promoting collagen orientation10. Hence, the actual roles of SP in the pathogenesis of tendinopathy remain controversial.
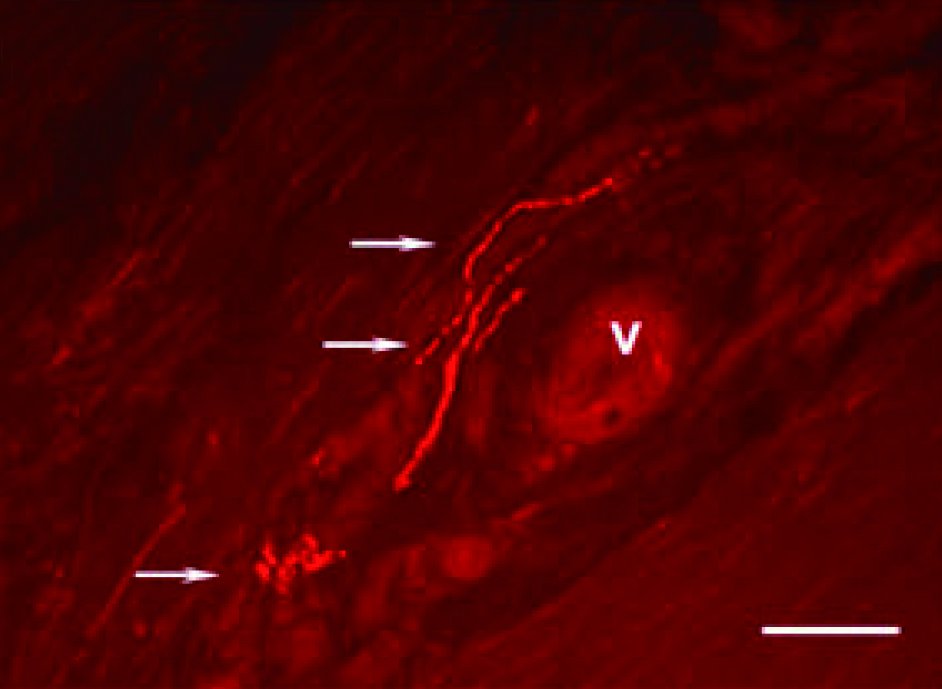
Figure 1. Immunofluorescence image of longitudinal sections from tendinosis tissue after immunostaining for SP2, Arrows: varicosities and nerve terminals, V: blood vessel, Bar: 50 microns (source of image: Ackermann PW, Int J Exp Pathol 2013)
Zhou et al. (2014) evaluated the effects of different doses of SP on tendon-derived stem cells (TDSCs) in vitro and rat tendons in vivo. The in vitro results showed that the proliferation ability of TDSCs was significantly enhanced in both low (0.1 nM) and high (1.0 nM) doses of SP (Figure 2). Interestingly, low-dose SP induced the expression of tenocyte-related genes, whereas high-dose SP induced the expression of non-tenocyte genes, as evident by the high expression of peroxisome proliferator-activated receptor γ (PPARγ) and collagen type II. In the in-vivo study, only high dose of SP (5.0 nmol) induced tendinosis-like changes in the tendon, while low-dose SP (0.5 nmol) enhanced the tenogenesis compared with saline injection and the high dose SP group11. According to the findings, the effects of SP on tendinopathy are likely dose-dependent.
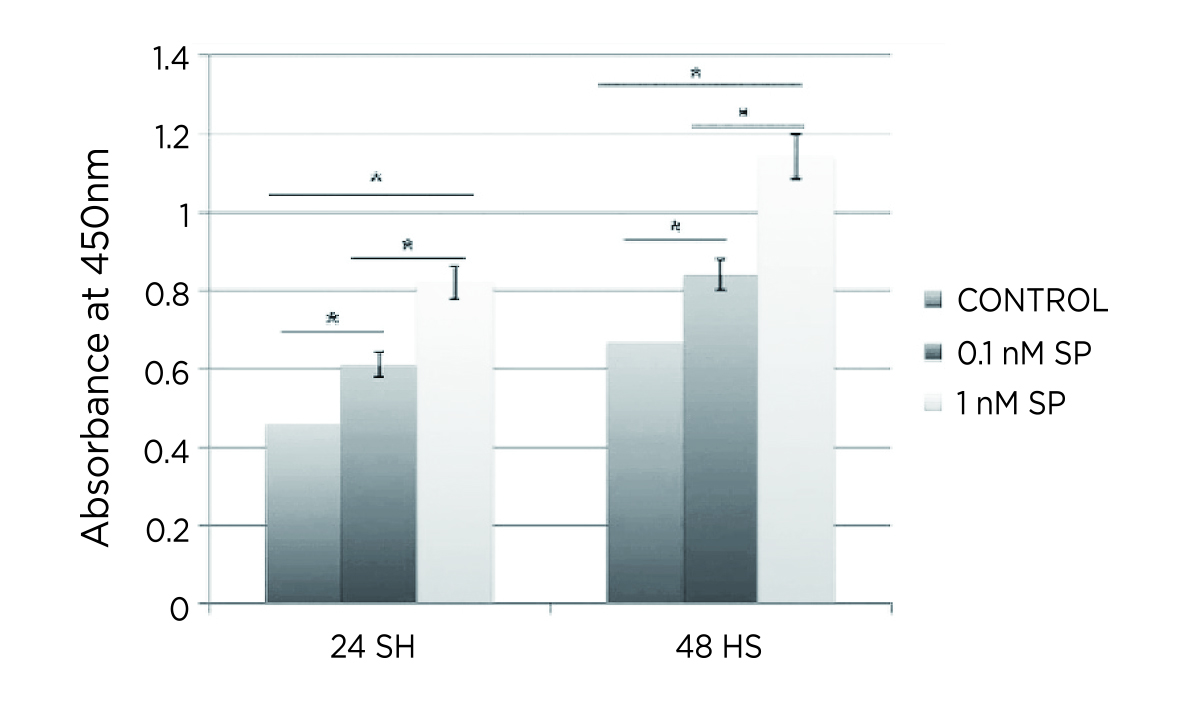
Figure 2. Significantly enhanced proliferation of TDSCs by SP11, *p<0.001
More recently, Oh and co-workers (2020) demonstrated in a rat model that injection of SP into Achilles tendons every other day for 14 days resulted in tendinopathic changes, including decreased tensile strength (Figure 3) and histological alterations3. These results suggested that sustained exposure to SP may be involved in the development of tendinopathy. Notably, this remark is supported by the findings in Zhou’s study above, given that a higher SP dose implies a longer half-life and, hence, a longer duration of SP exposure in tendons.
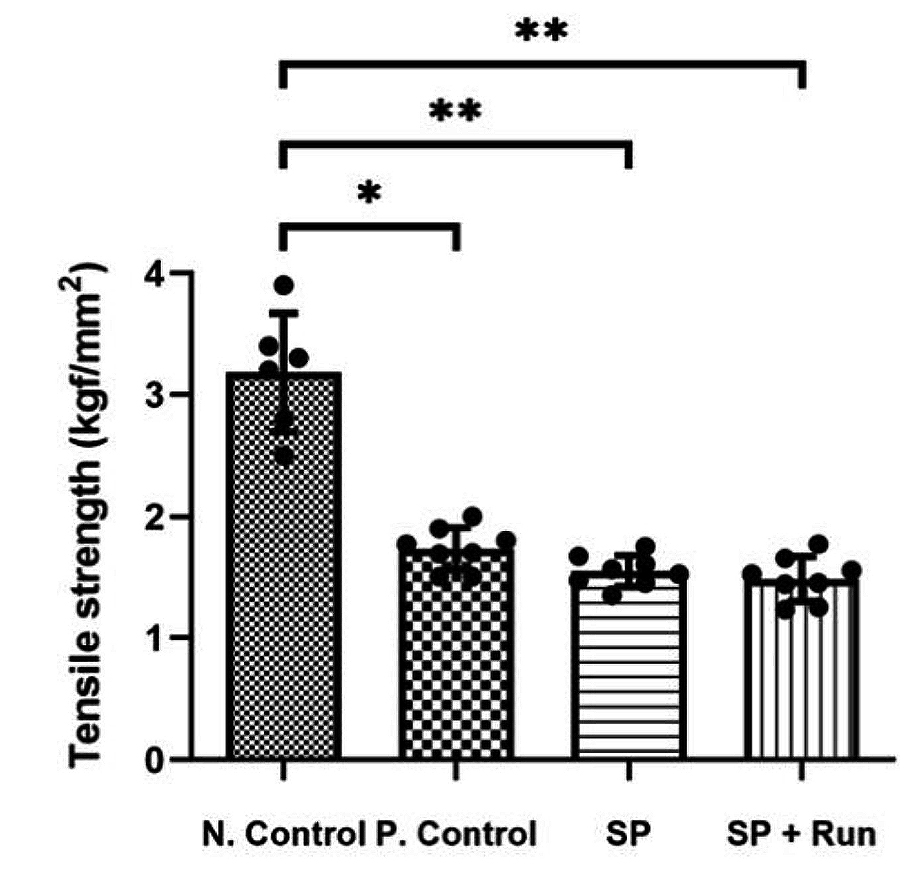
Figure 3. Comparison on tensile strength3, N.Control: negative control; P.Control: positive control; SP+Run: SP injection with treadmill running, *p<0.05 and **p<0.01
Provided that sustained SP exposure may be involved in the development of tendinopathy, investigations on the therapeutic potential of agents targeting SP in various musculoskeletal disorders are evoked. For instance,
Barbe et al. (2020) evaluated the outcomes of blocking SP- neurokinin 1 receptor (SP-NK1R) signalling with an antagonist (NK1RA) in a rodent overuse injury model. In the study, young adult rats were subjected to high repetition high force (HRHF) tasks for 3 weeks, with or without NK1RA treatment. The results showed a decline in grip strength in untreated HRHF rats (Figure 4), and mechanical sensitivity and temperature aversion increased compared to controls, whereas these changes were improved by NK1RA treatment. Importantly, NK1RA treatment reduced HRHF-induced thickening in flexor digitorum epitendons, and HRHF-induced increases of TGFbeta1, CCN2/CTGF and collagen type 1 in flexor digitorum muscles. Further, task-induced increases in collagen deposition in the forepaw upper dermis were reduced by NK1RA treatment12. The results confirmed that blocking the SP-NK1R signalling pathway with NK1RA may reduce several musculotendinous and dermal fibrogenic responses upon repetitive high-force lever pulling tasks.
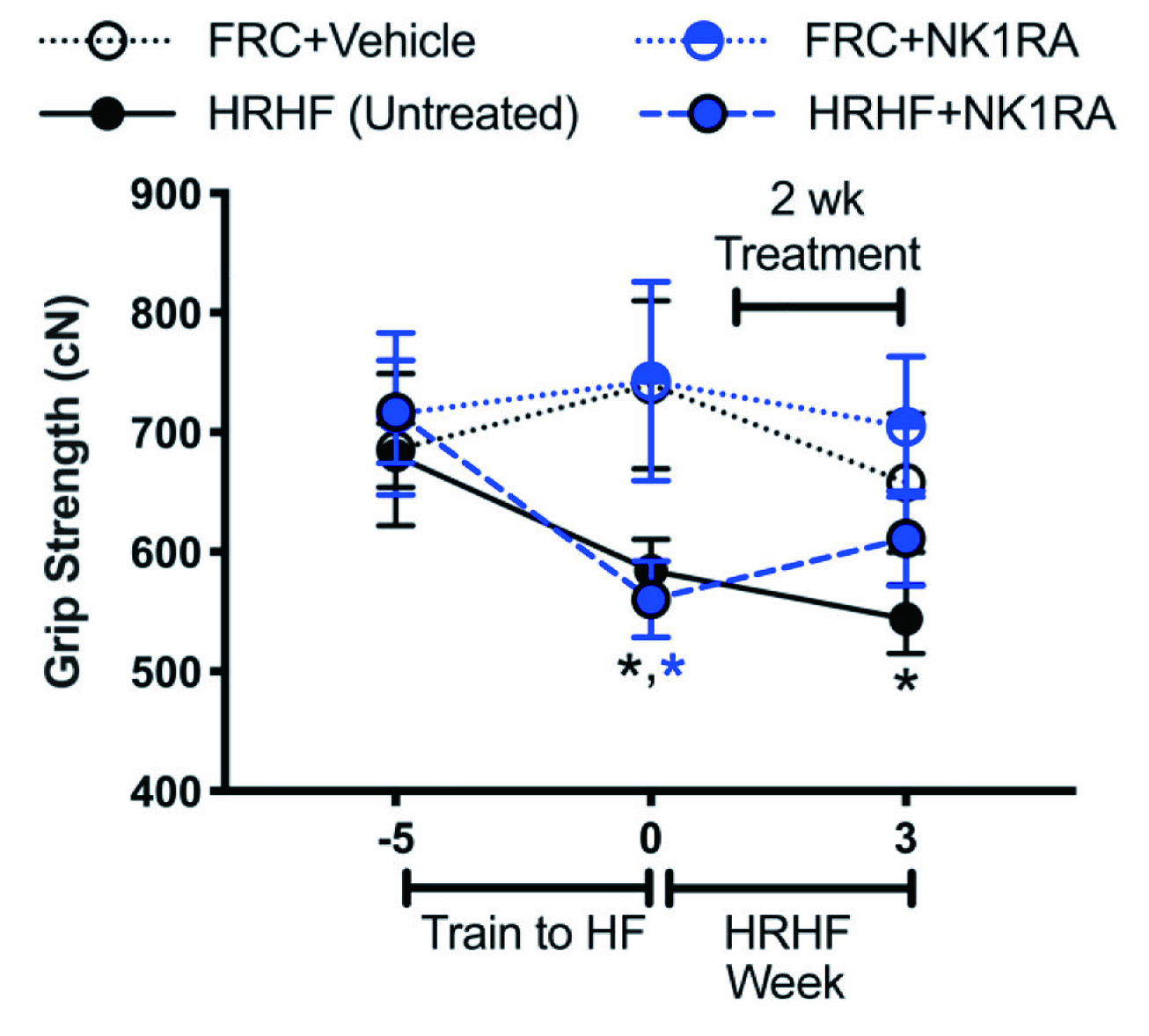
Figure 4. Functional assays in untreated HRHF, HRHF+NK1RA, and food-restricted control (FRC) rat groups treated with vehicle or NK1RA12, *p<0.05 against age-matched FRC group
Although the actual roles of SP in tendinopathy are still not fully understood, its involvement in both tendon healing in acute injury settings and the development of tendinopathic symptoms is evident. Apart from tendinopathy, there are studies reporting the roles of the SP-NK1R pathway in other musculoskeletal disorders, such as osteoarthritis. Thus, further works investigating the pathophysiological roles of the SP-NK1R pathway would provide insight for developing novel drugs countering tendinopathy and other diseases.
References:
1. Han et al. Int J Mol Sci 2017; 18. DOI:10.3390/IJMS18061241. 2. Ackermann. Int J Exp Pathol 2013; 94: 271. 3. Oh et al. Int J Mol Sci 2020; 21: 1–13. 4. Fong et al. J Orthop Res 2013; 31: 91–8. 5. Kujala et al. Clin J Sport Med 2005; 15: 133–5. 6. Tarantino et al. Int J Environ Res Public Health 2023; 20. DOI:10.3390/IJERPH20176681. 7. Jafari et al. Physiol Rep 2015; 3. DOI:10.14814/PHY2.12265. 8. Couppé et al. Clinical Biomechanics 2012; 27: 949–54. 9. Redkiewicz. Int J Mol Sci 2022; 23: 750. 10. Burssens et al. Foot Ankle Int 2005; 26: 832–9. 11. Zhou et al. J Nutr Health Aging 2015; 19: 555–61. 12. Barbe et al. Connect Tissue Res 2020; 61: 604–19.





