
NHS Specialist in
Northern Ireland, U.K.
Infectious diseases, including seasonal and emerging respiratory viral infections such as influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) have recently caused several epidemics and pandemics causing significantly disturbed daily life1. More, recently a new outbreak of a mosquito-transmitted virus has caused a havoc globally, particularly in developing nations where medical resources are limited. In the past, mosquito-borne diseases such as dengue fever typically occurred in tropical and subtropical regions causing up to approximately 100 to 400 million infections per year globally2. Nonetheless, the trend of this is changing as cases of dengue fever has been reported as far as United States (US), and Western Pacific, suggestive of a dengue fever epidemic attributed possibly by the global warming2,3. Locally, there are periodic outbreaks of dengue (Aedes aegypti and Aedes albopictus species) with around 153 cases confirmed since January 2018 to November 20184. To understand more about dengue fever, we have invited Dr. Haussam Elenin, one of the specialists in the Northern Ireland, United Kingdom (U.K.) to discuss more on the reasons for dengue fever spread, the disease burden in patients, and the current preventative measures taken to limit the impact of the disease.
The earliest outbreak of dengue was reported in Jakarta during the year 1779, Indonesia and the virus causing the disease was identified during the early 20th century5. Dengue has since become a global problem affecting individuals in over 120 countries and about half a million of those affected often requires hospital admission6. The worldwide cost of dengue is estimated to be USD 9 billion7, and in 2019, Philippines declared a national dengue epidemic with deaths rates reaching as high as 622 people annually8. So, what makes dengue infection so deadly? Dr. Elenin explains that dengue infection may affect several body systems, and in severe cases may impair the brain activities, resulting in altered level of consciousness in approximately 0.5%-7.4% cases9. Other neurological disorders such as transverse myelitis, and Guillain-Barré syndrome has also been reported in patients with dengue infections10.
Very recently, studies have shown dengue to predispose individuals to cardiovascular diseases (CVD) and pregnant females exposed to the virus are at higher risk of miscarriages, low birth weight (LBW) and premature births, particularly with dengue haemorrhagic fever (DHF)11,12. In recent decades, thanks to the uncontrolled expansion urbanisation, and increased population growth, dengue has now spread to a greater distance13. In fact, dengue fever was once confined only to Southeast Asia (71% cases), but now spreading as far as European14. According to recent epidemiologic studies, dengue is the leading cause of arthropod-borne viral disease in the world15. The World Health Organisation (WHO) considers dengue as one of the most neglected tropical disease10. Therefore, work is currently being done to eliminate the dengue vector, Aedes aegypti, and improve the current medical treatment which will target the virus directly17.
Dengue is an arboviral infection, also known as break-bone fever due to the severity of muscle spasms and joint pain which results during the viral incubation period15. Dr. Elenin explained that even though most cases are asymptomatic, severe illness and death may occur if left untreated, particularly with DHF. Moreover, literatures have shown individuals who were previously infected with one subspecies of the dengue virus may develop severe capillary permeability and bleeding after being infected with another subspecies of the virus, especially in patients with DHF15. Dengue fever has two transmission cycles that maintains the dengue virus, the first phase is where mosquito carries the virus from a non-human primate to a non-human primate, and the second phase is when the mosquito carries the virus from human to human (figure 1)15. “The human-mosquito cycle occurs primarily in urban environment and the transmission of human to mosquito is dependent on the viral load within the mosquito's blood meal,” according to Dr. Elenin. Infections are often asymptomatic in up to 75% of the cases and the disease maybe self-limiting, but without proper medical treatment, the mortality rate can exceed up to 20%15.
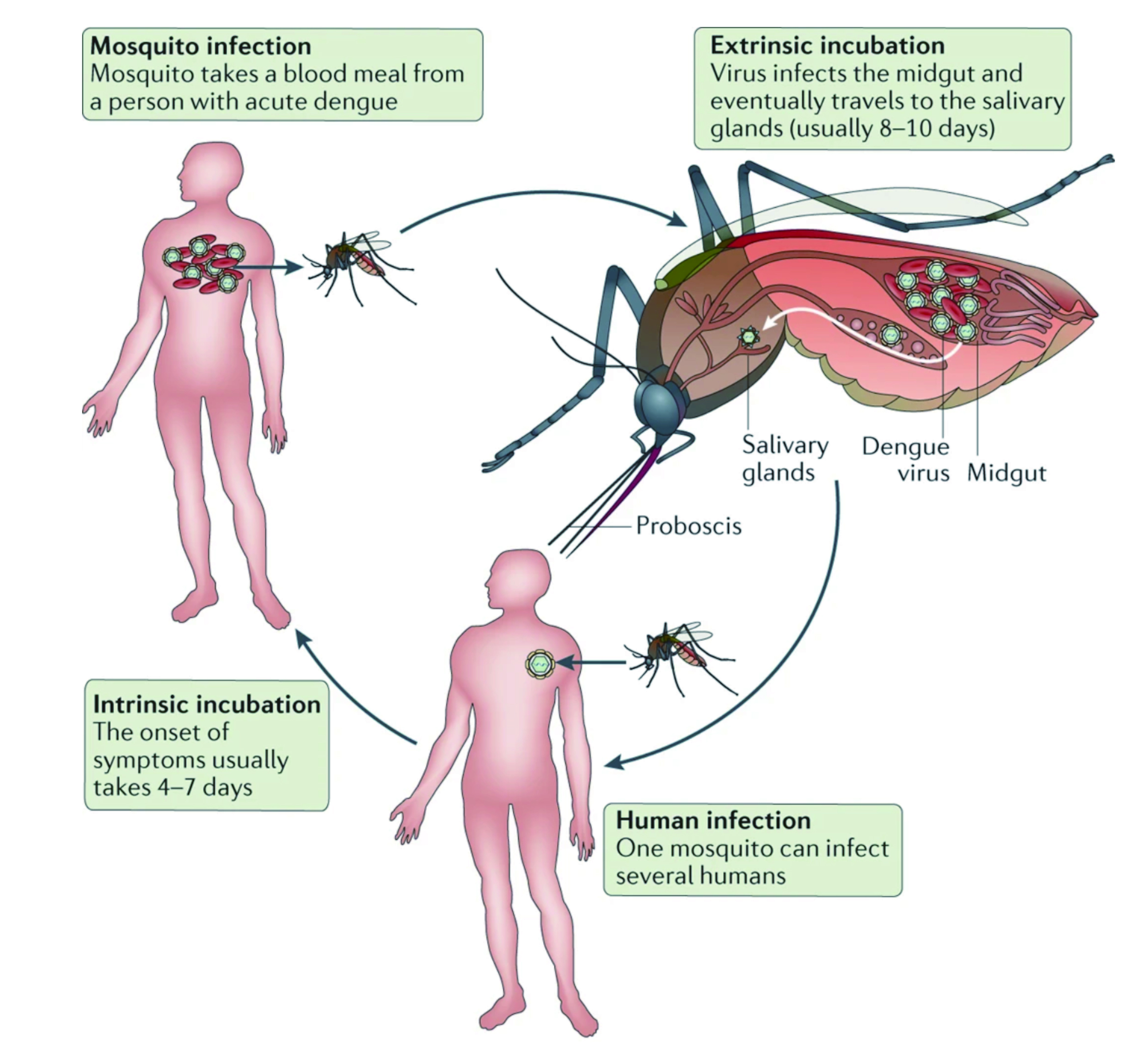
Figure 1. Dengue virus cycle in humans and mosquitos18
Notably, Dr. Elenin further explain that the disease primarily affects children and older adults, and the incubation period of the virus is around 4-7 days, but in some cases may last from 3 to 10 days, depending on the severity of the disease. He also emphasised that if symptoms last for more than two weeks after exposure, it is unlikely be due to dengue fever, therefore, an alternative diagnosis should be considered. There are three phases of dengue infection, which includes febrile, critical and recovery phases. During the febrile phase, a sudden high-grade pyrexia (40oC) occurs which lasts for two to seven days with biphasic pyrexia seen in 6% of cases15. “Some describe this as swinging fever, as individuals with dengue infection often have fever that weans and then spike on the following day for a few days,” as per Dr. Elenin. Patients may experience facial flushing, skin erythema, myalgias, arthralgias, headaches, sore throat, conjunctival infection, anorexia, nausea, and vomiting15. “The skin erythema in this case is blanching, more of macular, particular during the first few days of the infection, but in children, one has to remain vigilant and ‘look out’ for symptoms of septicaemia since this can potentially be missed19, and the results can be devastating,” Dr. Elenin emphasised. Pyrexia is also seen during the critical phase of the disease and associated with increased capillary permeability and thrombocytopenia with high haematocrit (Figure 2). It can progress to shock, organ dysfunction, or disseminated intravascular coagulation (DIC) or haemorrhage15. Therefore, “patient is at most risk during this phase and the mortality rate is usually high if patient is not managed correctly,” Dr. Elenin reiterated. Finally, the recovery phase is where there is a reabsorption of extravascular fluid and patient in this phase maybe experience symptoms of bradycardia20.
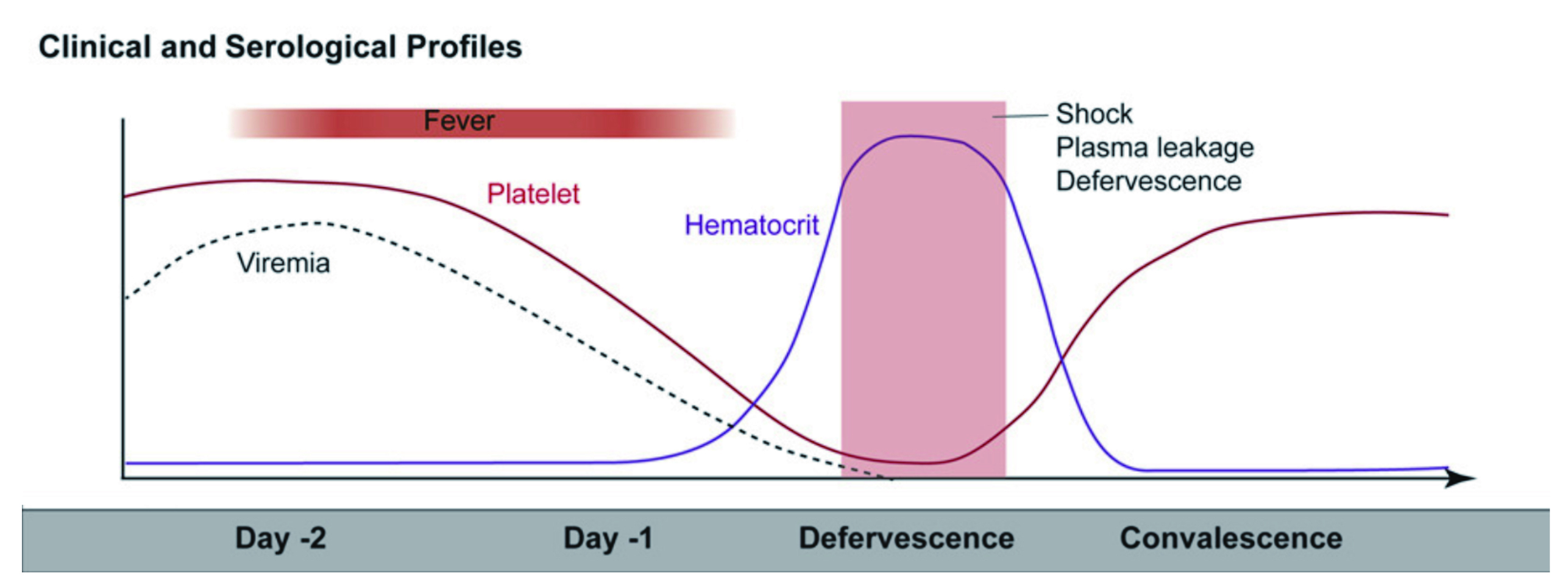
Figure 2. Clinical progression of dengue infection21
The clinical diagnosis of dengue can be challenging, and largely dependent on the stage of infective process in a patient. Moreover, there are other disease which may mimic dengue19, therefore, it is important to take appropriate travel history, Dr. Elenin suggested. The ambiguity in diagnosing dengue infection has also been highlighted in literatures as the early stages of the disease may present as a mild undifferentiated “flu-like” fever with symptoms which are similar to influenza, measles, Zika, chikungunya, yellow fever and malaria19 Since the clinical diagnosis of dengue is so diverse, it is often a challenge to diagnose the condition accurate, and essential laboratory analysis such as reverse-transcription polymerase chain reaction (RT-PCR), and NS1 antigen-capture enzyme-linked immunosorbent assay (ELISAs) are helpful tools used for a more rapid diagnosis19. In fact, the NS1-based testing has shown to have a high positive and negative percentage agreement (92.8 positive percentage agreement [PPA] and 91.7% negative percentage agreement [NPA]) in acute setting within 0-5 days of symptoms onset, thereby increasing the accuracy of diagnosis in endemic areas22.
Since dengue also affects other organs, the cardiovascular (CV) manifestations are no exception with 12.5% of CV complications reported in patients with severe dengue11. “Nevertheless, the clinical data on this is scarce and cases are often underreported”, according to Dr. Elenin. Recently, the Neglected Tropical Diseases and other Infectious Diseases involving the Heart (NET-HEART) project aimed to expand the knowledge on CV involvement of infectious disease and to help identify barriers to diagnosis and treatment11. Data showed a low prevalence of CVD in dengue infections, highlighting poor reporting or documenting strategies, which inadvertently leads to poor performance of most diagnostic test11. Furthermore, dengue may also cause cardiac arrythmias, hypotension, myocarditis, pericarditis, and heart failure (HF)11,23. The recent shift of dengue paradigm may well be a result of global warming since we are now seeing dengue cases even in developed nations and this shift is exposing individuals who have never been exposed to the virus, Dr. Elenin commented. Therefore, like SARS-CoV2, those who had never been exposed to the virus are at risk of developing severe disease, especially those who are immunocompromised, as well as the older adults23.
The precise mechanism involved in the increased risk of HF by dengue infection remains elusive. Some studies have shown the virus to cause direct infection of the cardiomyocytes and endothelial cells of the blood vessels, leading to myocarditis and vasculitis23. On this note, Dr. Elenin suggested the acute infection by dengue virus triggers the activation of the sympathetic nervous system, which may put enormous strain on the heart since the CV health is already compromised by the increased capillary permeability. Thereby, with the interstitial oedema, the preload is significantly reduced, affecting the microcirculation, and giving a clinical picture of hypovolaemic shock (Figure 3). The already compromise CV system may then reach a critical point, leading to acute heart failure (AHF), especially among individuals with already compromised cardiac function or those with pre-existing CVD23.
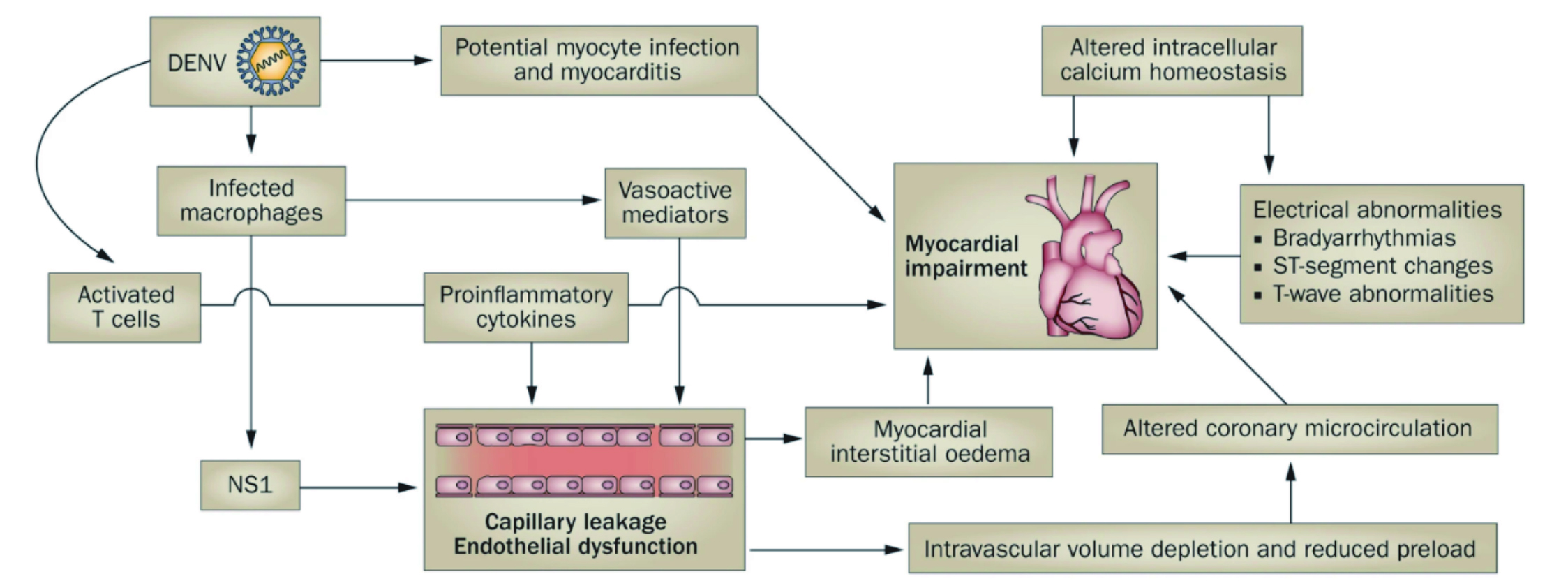
Figure 3. The proposed viral and immune mechanisms that leads to cardiac and vascular manifestations of dengue24
A recent observational study by Wei et al., in 2022 suggested major acute cardiovascular events (MACEs) are highest in patients above the age of 60 with a higher prevalence seen in females compared to males. Furthermore, the MACE risk was nearly six times higher among admission cases of dengue, compared to non-admission cases. The incidence rate ratio (IRR) of MACEs was 17.9 (95% CI, 15.80-20.37) during the first week after onset of the infection. Moreover, the IRR related to HF was 27.24 (95% CI, 22.67-32.73; p<0.001)25.
Dr. Elenin advocated to closely monitor patients with dengue infection since dengue infection may predispose them to HF or other CV related comorbidities. To put this into perspective, Dr. Elenin shared a case of a 73-year-old male on Apixaban with atrial fibrillation (AF) admitted secondary to DHF. Patient noted to have travelled to Brazil two weeks prior to the symptoms and became acute unwell upon his arrival back to the United Kingdom (UK). Patient also reported increasing lethargy, with swinging fever, and symptoms were suggestive of severe dengue infection. Nonetheless, during the second day of his admission, he began to experience dyspnoea, orthopnoea, and noted to have significant lower limb swelling, in addition to deteriorating renal function. Patient had no documented symptoms of HF and his AF has always been well controlled. Upon close examination and ECG monitoring, significant conduction anomalies were seen, and patients was referred to the cardiology team who diagnosed him with AHF with ongoing severe acute kidney injury (AKI). Fortunately, his symptoms were promptly treated, and he made an uneventful recovery. Nonetheless, this case highlights that importance to monitor early and take a multidisciplinary approach when treating patients with infectious disease such as dengue since things may escalated very quickly. In conclusion, dengue infection can cause multiorgan failure and considerable percentage of patients, especially children and older adults may require hospital admission. Moreover, the cardiac complications can sometimes be overlooked and underdiagnosed in clinical practice which may contribute to the mortality observed with dengue infections. Finally, Dr. Elenin encouraged clinician colleagues to remain vigilant and take a stepwise approach when treating the disease to ensure patients safety is not compromised and they receive the most appropriate care.
References
1. Ryu S, et al. Viruses 2022; 14(11). 2. Kok BH, et al. Virus Res 2023; 324: 199018. 3. Rocklöv J, et al. Emerg Top Life Sci 2019; 3(2): 133-42. 4. Chan EYY, et al. PLoS Negl Trop Dis 2021; 15(1): e0008993. 5. Guo C, et al. Front Cell Infect Microbiol 2017; 7: 317. 6. Tangsathapornpong A, et al. PLOS Glob Public Health 2023; 3(2): e0001558. 7. Stephenson C, et al. Insects 2022; 13(2). 8. Dyer O. BMJ 2019; 366: l5098. 9. Carod-Artal FJ. Res Rep Trop Med 2014; 5: 95-104. 10. Rodríguez Y, et al. Cell Mol Immunol 2018; 15(6): 547-62. 11. Araiza-Garaygordobil D, et al. Cardiovasc J Afr 2021; 32(5): 276-83. 12. Paixão ES, et al. BMJ Open 2019; 9(7): e023529. 13. Kyle JL, et al. Annu Rev Microbiol 2008; 62: 71-92. 14. Gossner CM, et al. Euro Surveill 2022; 27(2). 15. Schaefer TJ, et al. StatPearls Publishing LLC.; 2023. 16. Molyneux D. Community Eye Health 2013; 26(82): 21-4. 17. Buhler C, et al. PLoS Negl Trop Dis 2019; 13(7): e0007420. 18. Guzman MG, et al. Nature Reviews Disease Primers 2016; 2(1): 16055. 19. Muller DA, et al. The Journal of Infectious Diseases 2017; 215(suppl_2): S89-S95. 20. Zerfu B, et al. Trop Med Health 2023; 51(1): 11. 21. Arora JK, et al. iScience 2022; 25(4): 104034. 22. Versiani AF, et al. Diagnostics (Basel) 2023; 13(6). 23. Wei KC, et al. Travel Med Infect Dis 2023; 53: 102589. 24. Yacoub S, et al. Nature Reviews Cardiology 2014; 11(6): 335-45. 25. Wei KC, et al. PLoS Negl Trop Dis 2022; 16(2): e0010134.





