
Clinical Assistant Professor,
Department of Dermatology and Skin Science,
University of British Columbia, Canada
Probity Medical Research - Principal Investigator
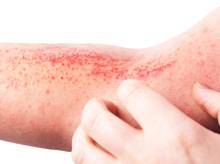
Atopic dermatitis (AD) is a chronic relapsing and remitting disease characterised by flares or exacerbations over years. The disease poses a significant burden on individual quality of life and the healthcare system.1,2 Timely and effective management of AD would relieve the suffering of patients and its impacts on the healthcare system. A treat-to-target (T2T) framework is essential in guiding decisions on disease assessment and subsequent treatment for the optimisation of patient outcomes. In the web-based clinical workshop titled “Treat-to-Target in AD Management” held on 3rd October 2021, Dr. Chih-ho Hong from the University of British Columbia shared his insights in managing AD and highlighted the development of consensus recommendations on T2T approach for systematic treatment in adults with moderate-to-severe AD in the real world setting.
Treat-to-Target in AD Management:
Global Consensus Guiding Treatment Journey
AD at a glance
AD affects up to 5% of the global population, whereas the prevalence among children can be up to 20% in certain countries.1,2 Essentially, while more than 30% of AD patients have reported to have moderate-to-severe disease,1 suboptimal long-term outcomes are common among these patients due to the absence of effective systemic treatment.3
Despite the pathophysiology of the disease is not yet fully understood, it is clear that AD is an immune-mediated type 2 inflammatory disease characterised by the dysregulation of T helper 2 pathway and enhanced penetration of allergens into the skin.4,5 Type 2 cytokines of IL-4 and IL-13 both act a key role in the pathogenesis of AD, the mechanism is complex and it can result from genetic, environmental as well as immunological factors.4,6,7
For patients suffering from AD, there is no wonder that comorbidity with other type 2 inflammations comes relatively common, such as food allergy, allergic rhinitis, and asthma.8 It poses multidimensional burden to the patients as well, including sleep disturbance, psychosocial well-being, and quality of life; whereas their families are also involved.9,10 AD patients reporting pruritus are more likely to experience suicidal ideation, mental health problems, and mental distress as compared to those without the condition.11 Thus, the widespread impacts of AD have to be taken into account in addition to clinical manifestations when managing the disease.
Clinical assessment of AD
Consistency in outcome measurement is important for the advancement of clinical research and patient management. HOME, a global initiative incorporating relevant stakeholders, has set out four core outcome domains to be measured and reported in all AD clinical trials (Table 1). While various assessment tools/instruments for one or more of the domains are available, the HOME initiative recommends some instruments which are the most appropriate for each domain. For example, the Eczema Area and Severity Index (EASI) and Atopic Dermatitis Control Test (ADCT) are recommended for clinician-reported signs and long-term control, respectively.12
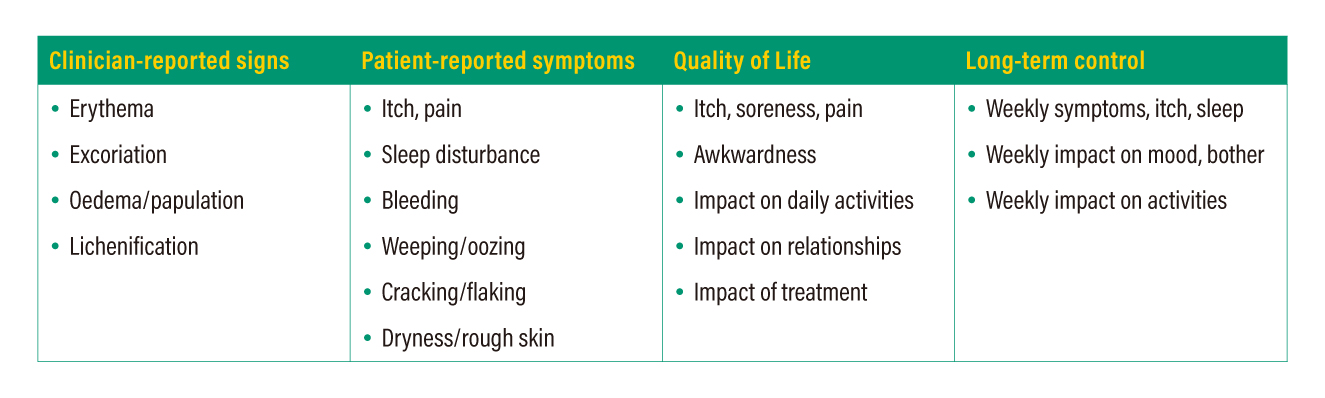
Table 1. Core outcome domains to be reported in clinical trials on AD
Treat-to-target in AD
Most current guidelines provide a list of treatment options according disease severity/activity with minimal recommendation on how to optimise and to measure for treatment success. Without a proper standard, patients may not enjoy the maximal benefits out of the management strategy necessary for optimal disease control.13 T2T is an established treatment strategy in which any treatment performed is oriented by explicitly specified and sequentially measured goals (such as remission or low disease activity for the case of rheumatoid arthritis).13,14 For patients with moderate-to-severe AD, the first T2T framework has been established recently to guide systemic treatment particular for the optimisation of therapy in the first 6 months.
The framework encompasses 16 recommendations spanning 3 areas (guiding principles, decision-making, and outcome thresholds), and consensus on all of them was reached within an international panel of dermatologists, dermatology nurses, and patient representatives after 2 eDelphi rounds. Each recommendation statement was rated using a 9-point Likert scale ranging from 1 for “strongly disagree” to 9 for “strongly agree”; once 75% or more of all participants had rated their agreement level as ≥7, consensus agreement was reached.13
The guiding principles summarise on the delivery of patient-centred care management with an emphasis on the assessment of changes in disease severity/impacts and treatment safety/tolerability; while the decision framework give pragmatic recommendations informing actions to be taken at two specific time points (3 months and 6 months). For outcome thresholds, treatment target goals are listed with clear and explicit guidance. Highlighting shared decision-making and patient satisfaction, a decision-making algorithm is developed (Figure 1). Changes in the patient’s global assessment and changes in at least one specific disease domain measured with the use of validated outcome assessment instruments shown in Figure 1 act as a ground for decisions for continuing or changing therapy.13 Flexibility is allowed on the choice of disease domain at the time of assessment. Nonetheless, treatment safety and tolerability are of cardinal considerations and should be taken into account at each decision point.
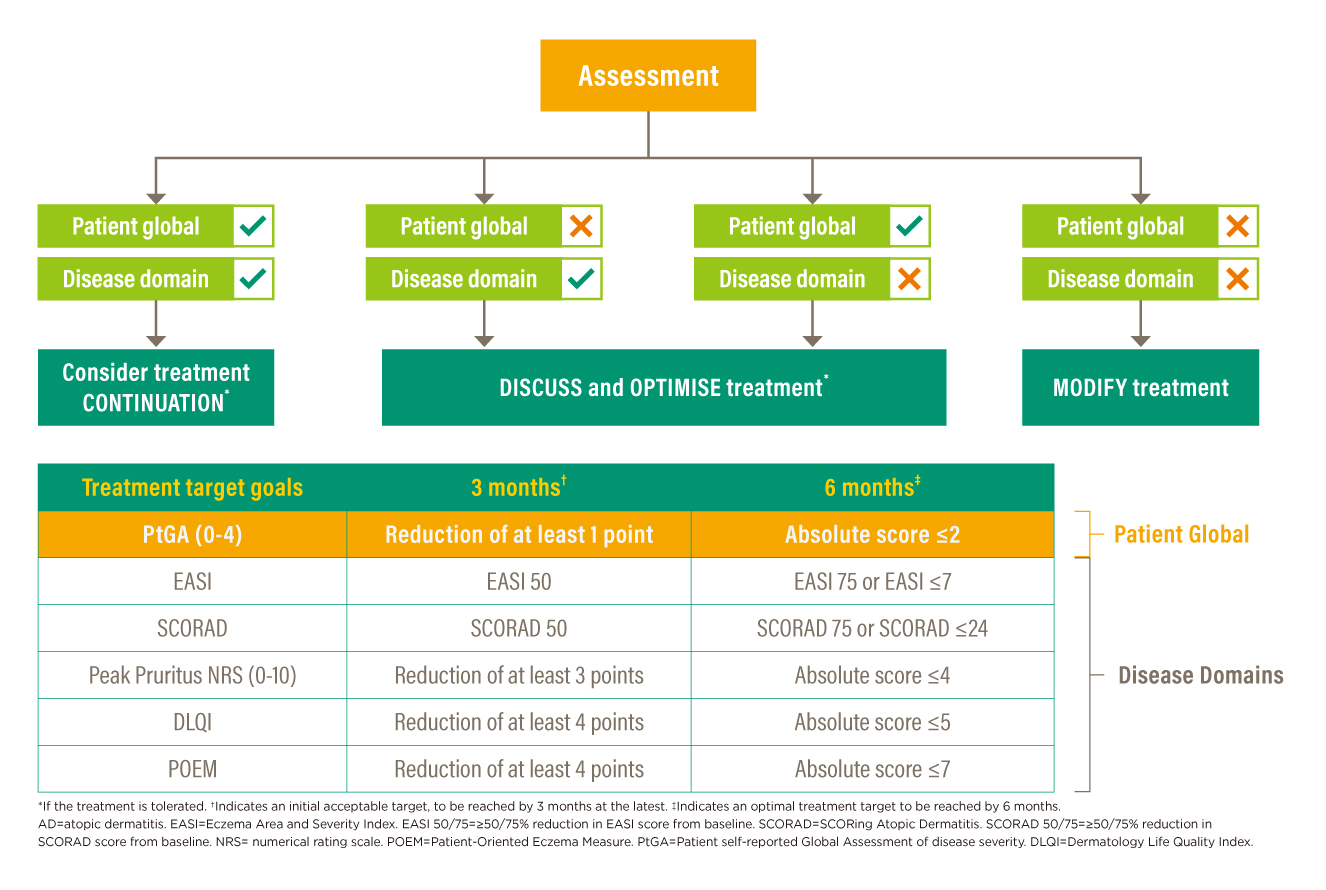
Figure 1. AD T2T decision-making algorithm
Eczema Area and Severity Index
EASI is a composite index generally adopted in assessing patients with mild-to-severe AD, its manipulation involves the conversion of clinical judgement of disease extent and affected body surface area to a score representing severity in a single patient at one time. Defining severity in numerical terms enables the detection of change in the patient so far in a more scientific way which then informs about efficacy of treatment employed.15
The calculation of score is a repetition of 2-step procedure. First, evaluator assesses the disease extent and percent of affected body surface area on a scale of 0 to 6 (0 for 0%, 1 for 1-9%, 2 for 10-29%, 3 for 30-49%, 4 for 50-69%, 5 for 70-89%, and 6 for 90-100%) in selected body region (head/neck, trunk (including the genital area), upper extremities, and lower extremities (including the buttocks)). Severity of each of the four signs (erythema, oedema/papulation, excoriation, and lichenification) in the specific body region is then graded on a scale of 0 to 3. As each body region has its respective multiplier, the region score is obtained by multiplying the sum of severity score, area score, and the multiplier (0.1 for head/neck, 0.3 for trunk, 0.2 for upper extremities, and 0.4 for lower extremities). The final EASI score is the sum of all four region scores, ranged from 0 to 72.15,16 The interpretation of EASI is based on a 6-strata severity (0 for clear, 0.1-1 for almost clear, 1.1-7 for mild, 7.1-21 for moderate, 21.1-50 for severe, 50.1-72 for very severe).17 Yet, even with identical scores, the manifestations displayed can be varied in terms of phenotypes, severity, and extent. The evaluation of EASI can be subjective and inter-evaluator variability can occur. Hence, it is recommended to perform assessment on a given patient throughout the treatment course by the same evaluator.18
Atopic Dermatitis Control Test
ADCT is a newly developed instrument aiming to assess patient-perceived disease control and foster patient-physician communication around the topic. There are six items in the overarching measurement of disease control, including overall severity of symptoms, number of days with intense episodes of itching, intensity of bother, problem with sleep, impact on daily activities, and impact on mood or emotions. The assessment could be utilised from baseline to a much longer term measurement of AD control.
The assessment involves a self-rated test by the patient on the severity of each item on a scale ranged between 0 (no problem) and 4 (very severe) within a defined 7-day recall period. The sum of scores of all items reflects the status in disease control, whereas a higher score indicates poorer disease control (Table 2). For instance, a total ADCT score of ≥7 implies the patient’s AD condition is not in control.
Apart from indicating disease control, the instrument would illustrate the chronical changes in treatment outcomes upon repeated assessment, with a threshold of 5 points is considered as clinically meaningful within-person change. However, the applicability of ADCT is currently limited to adult patients only; ongoing research is in process to test and validate ADCT in the paediatric AD population.19
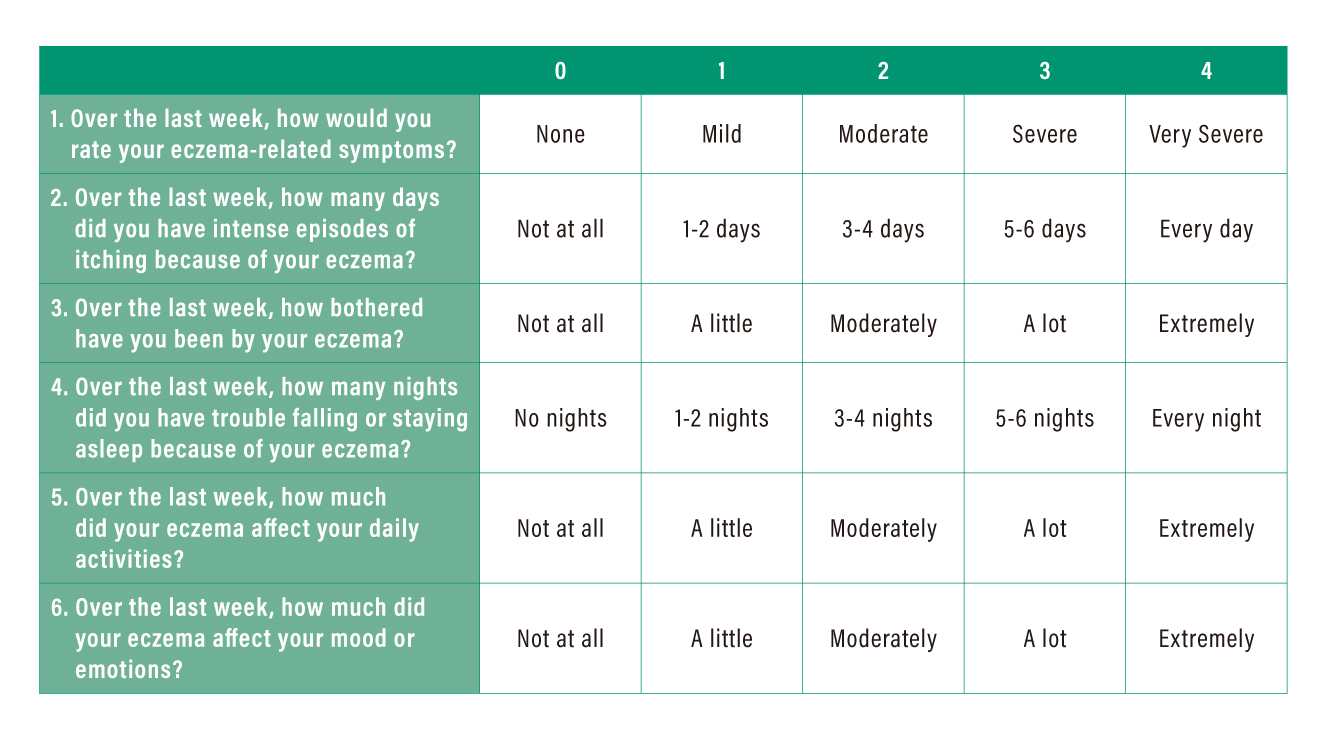
Table 2. Definition of severity score in ADCT*
The ADCT has gone through item-level evaluation with other existing patient-reported outcome measures and demonstrated strong correlations between the end scores which confirmed construct validity for the ADCT total score. The mean ADCT total scores matched with the AD control Patient Global Assessment (PGA) strata, especially the mean total scores for “mostly controlled” and “a little controlled” were below and above the threshold of ≥7 points respectively, representing its ability to differentiate between degrees of disease control. Similar results were obtained for the case of Patient Oriented Eczema Measure (POEM) severity subgroups, the mean ADCT total scores were 4.18 for “none or mild”, 9.71 for “moderate”, and 16.34 for “severe or very severe”.19 The ADCT equips sufficient discriminating ability to inform clinical decision-making.
In view of its user-friendliness, the ADCT can be easily incorporated into clinical practice. While the test is based on self-reported data by patients, computation of ADCT score is relatively straightforward. The easy interpretation of total ADCT score and its trend would facilitate meaningful patient-physician conversations, and hence enables shared decision-making and improvements in clinical monitoring.
The development of assessment instruments/tools allows evaluation on different aspects of the disease. Nonetheless, in view of the specific scope and limitations of each instrument, a combination of them would help to capture more desirable information for physicians to better understand the patients’ conditions.
T2T in the Real World
To demonstrate the practice of T2T approach in clinical settings, Dr. Hong presented the cases of two AD patients.
CASE SHARING 1
In the first case, the 18-year-old male patient was of mixed Japanese/Caucasian ancestry with strong family history of AD in the Caucasian side. The patient did not respond well to initial treatments and his parents refused the suggested treatment with immunosuppressants. The patient was then prescribed with dupilumab. At week 16, EASI 50 achieved and ADCT total score was lowered to 10, indicating a clinically meaningful within-person change. Yet, both Investigator’s global assessment (IGA) and Patient self-reported global assessment (PtGA) were still rated as “moderate”. Although reductions were noticed in EASI, ADCT and Peak Pruritus NRS, there was no improvement in his PtGA. Therefore, the T2T assessment algorithm was referenced for the optimisation of treatment. The patient discussed with the attending physician and subsequently started a 3-month trial of weekly dupilumab in conjunction with topical steroids optimised and phototherapy being added back. At the end of trial (week 28), EASI 75 was achieved and PtGA became “mild”.
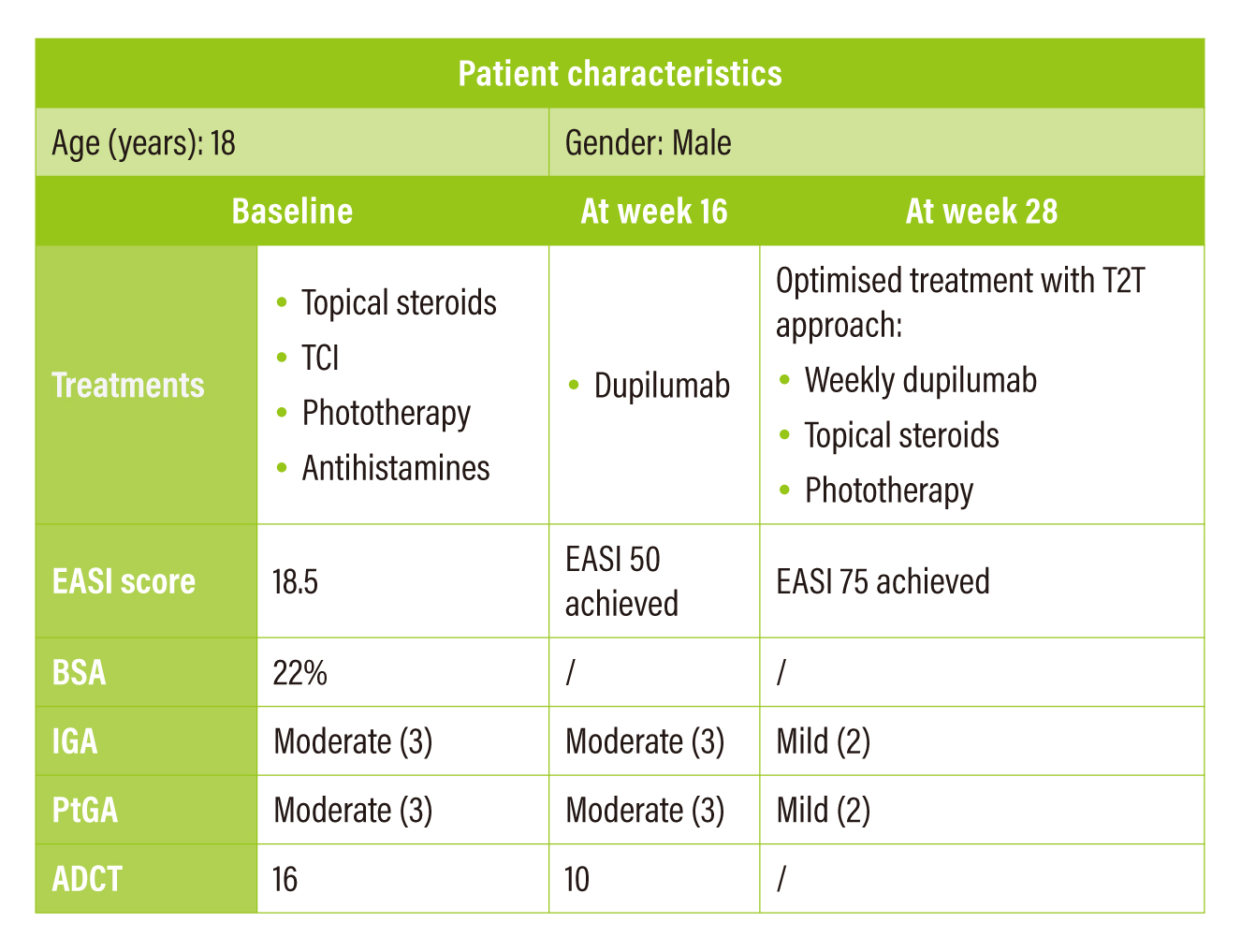
Table 3. Summary of case sharing 1
CASE SHARING 2
The patient was a 23-year-old male of Chinese ancestry with childhood onset AD. He was initially treated with cyclosporine 100 mg twice a day for 3 months, whereas the treatment yielded only modest improvement of a drop of 4 points in EASI score while the scores of body surface area (BSA), IGA, PtGA and ADCT remained unchanged. The ADCT total score of 20 indicated poor disease control in the patient even with cyclosporine at optimal dosing. The T2T assessment algorithm indicated to modify existing treatments. Then, the patient switched to dupilumab in accordance with the recommended dosing. At week 24, the new treatment brought significant improvement. There was an overall decrease in all assessment scores (EASI: 4.5; BSA: 2%; IGA: Almost clear (1); PtGA: Clear (0); and ADCT: 3). A drop of ADCT total score to below 7 suggesting that the disease was in good control.
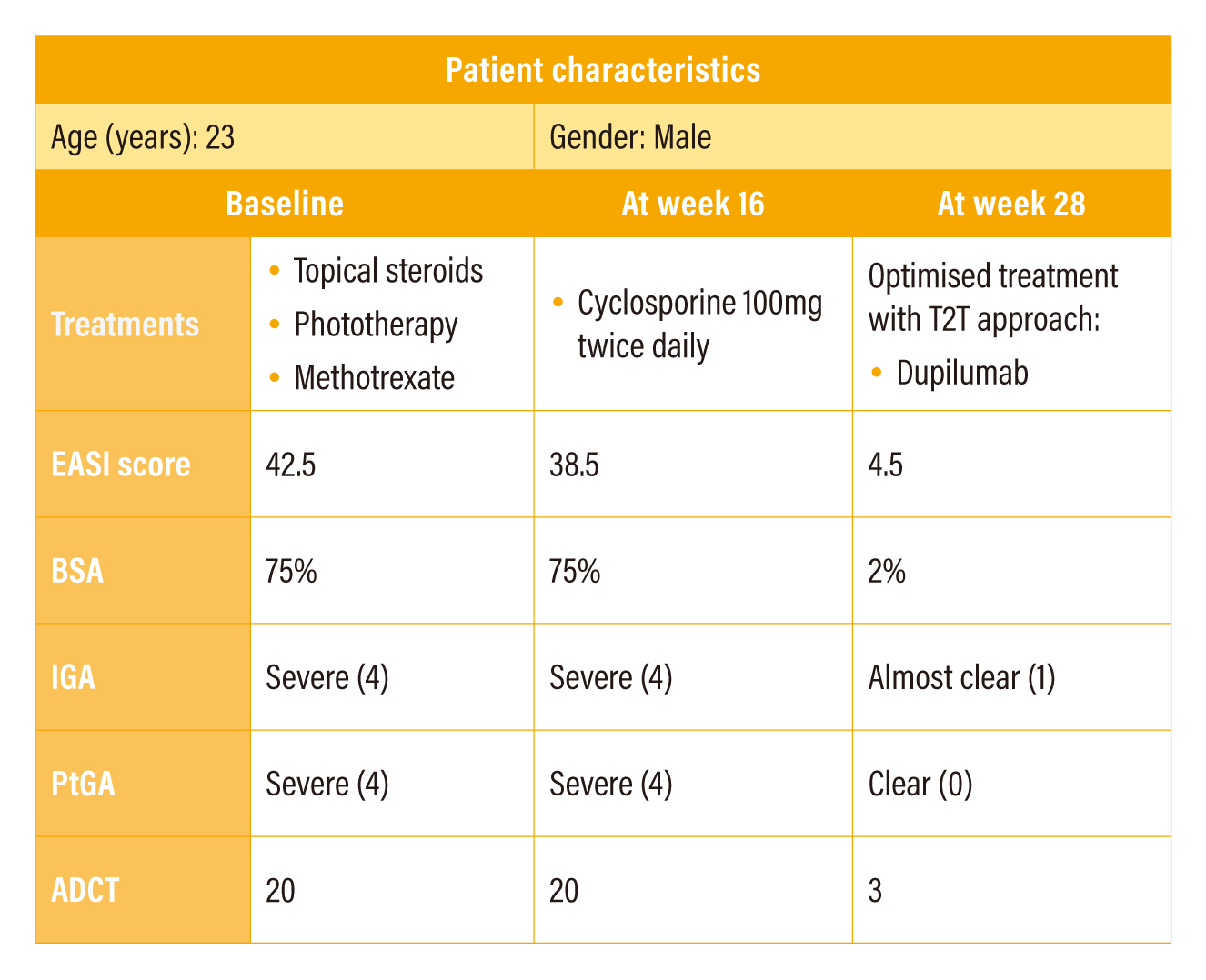
Table 4. Summary of case sharing 2
References
1. Barbarot S, et al. Allergy. 2018;73(6):1284-1293. 2. Nutten S. Ann Nutr Metab. 2015;66 Suppl 1:8-16. 3. Guttman-Yassky E, et al. Expert Opin Biol Ther. 2013;13(4):549-561. 4. Cabanillas B, et al. Curr Opin Allergy Clin Immunol. 2017;17(4):309-315. 5. Paller AS, et al. J Am Acad Dermatol. Published online October 28, 2021. 6. Akdis CA, et al. Allergy. 2020;75(7):1582-1605. 7. Peng W, Novak N. Clin Exp Allergy. 2015;45(3):566-574. 8. McCormick JP, Lee JT. J Inflamm Res. 2021;14:4259-4266. 9. Simpson EL, et al. J Am Acad Dermatol. 2016;74(3):491-498. 10. Drucker AM, et al. J Invest Dermatol. 2017;137(1):26-30. 11. Halvorsen JA, et al. J Invest Dermatol. 2014;134(7):1847-1854. 12. Harmonising Outcome Measures for Eczema (HOME). Core Outcomes for trials (Accessed on 30 November 2021). Available at http://www.homeforeczema.org/research/index.aspx 13. De Bruin-Weller M, et al. Acta Derm Venereol. 2021;101(2):adv00402. 14. Solomon DH, et al. Arthritis Rheumatol. 2014;66(4):775-782. 15. Rullo VEV, et al. Allergol Immunopathol (Madr). 2008;36(4):205-211. 16. Harmonising Outcome Measures for Eczema (HOME). EASI (with instructions). (Accessed on 30 November 2021). Available at http://www.homeforeczema.org/documents/easi-user-guide-jan-2017-v3.pdf 17. Thyssen JP, et al. Dermatol Ther (Heidelb). 2021;11(3):845-853. 18. Hanifin JM, et al. Exp Dermatol. 2001;10(1):11-18. 19. Pariser DM, et al. Curr Med Res Opin. 2020;36(3):367-376.





