
Medicated Contact Lenses the Next Innovative Ocular Drug Carrier System
The eye is a complex yet delicate organ, providing us with one of the most important aspects of life, the power of sight. Though if ocular diseases are left untreated, it can negatively impact a patient’s quality of life1. This article will turn towards contact lenses as the new innovative medication for treating eye diseases. Bringing medicated contact lenses into the market put forward a new perspective on helping patients adhere to their treatment. As well as enhancing bioavailability, reducing undesired symptoms and preserving patients’ ability to see in the long run.
Do You See the Problem?
Eyes are prone to dryness, irritation and various diseases. According to the World Health Organization (WHO), every five seconds, a person in the world becomes blind1. In 2040, about 112 million people will be affected2. Some of the most common ophthalmic diseases are dry eyes, conjunctivitis, glaucoma, uveitis and cataract1,3. If left untreated, these ocular conditions can negatively affect patients’ quality of life1.
90% of marketed ophthalmic drugs available in the industry are administered as eye drops3, which is highly accepted by the public1,3. Long drug-cornea contact time is required for efficient drug delivery, allowing high corneal permeation. The bioavailability of topical eye drops is very low, considering nasolacrimal drainage and reflex blinking, 20% (7 £gL) of the instilled dose is retained in the precorneal pocket3. Only a maximum of 5% of the active drug can reach the deeper ocular tissues1.
Moreover, patients with glaucoma were found to have poor medication adherence. In the first 18 months after patients were diagnosed with glaucoma, around 20% did not visit their ophthalmologist. Furthermore, only 10% of glaucoma patients ordered repeat prescriptions timely within 12 months, which suggests that they are non-compliant with their medication and not adhering to the treatment regime. Non-adherence with glaucoma medications has a reported rate of 16%-30%4.
Contact Lens as the Vehicle for Drug Delivery
The most potential ocular drug delivery system other than eye drops are contact lenses. Around 100 million people are wearing contact lenses, and it will only increase exponentially in the near future1. Contact lenses are made of hydrogel, and they can adhere to the tear film due to surface tension. Making them an excellent candidate to act as a direct vehicle to deliver medication and resolve the limitation of eye drops, reaching a more sustained drug release. By reducing the frequency of administration and dose required to achieve the desired therapeutic effect, prolonging contact time on the post lens tear film1. Contact lenses can increase drug retention on the cornea surface up to 30 minutes, providing a potential 50% drug bioavailability2.
Theoretically, drugs would be loaded onto the contact lenses by soaking in drug solution. Though soaked contact lenses have the disadvantage of low drug loading, drug release duration is still inadequate. To overcome these limitations, researchers have looked into a few methods to improve the control of drug release and drug loading capacity (Figure 1).
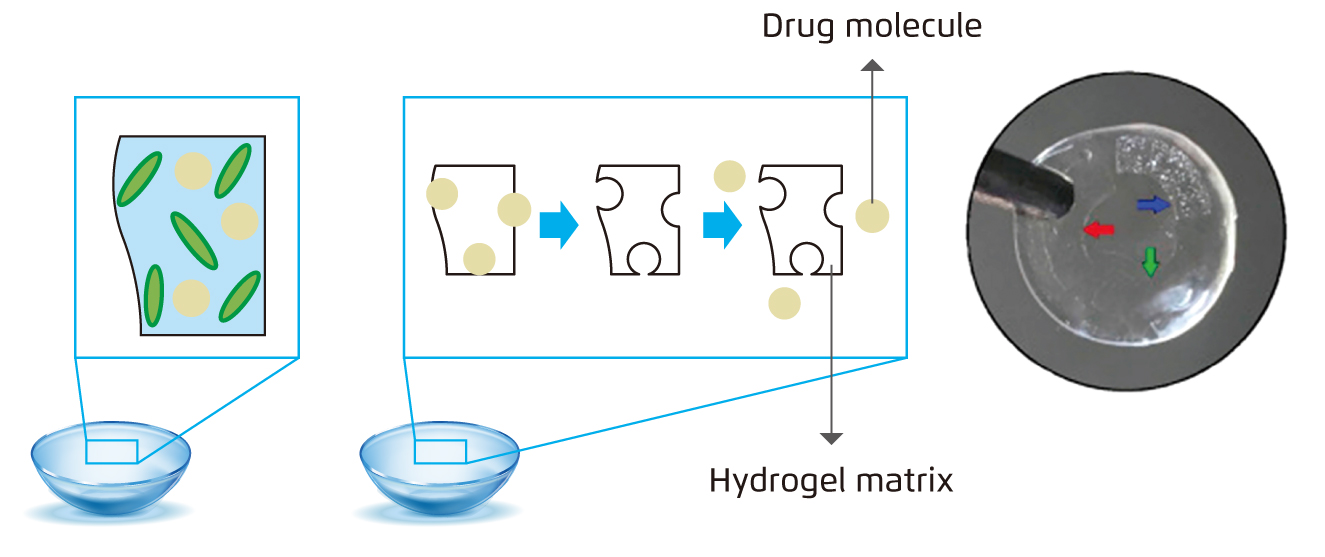
Figure 1.
Representative image of the different methods to load drugs into soft contact lenses: (a) Bulk barriers5 (b) Molecularly imprinted contact lenses5 (c) Implanted contact lenses (highlighted by red, green, and blue arrows) 6
Extending Drug Release Duration
Researchers have suggested adding a physical barrier into the contact lens matrix (Figure 1a). Aiming to extend the drug release time without compromising the contact lens’ transparency, oxygen permeability, ion permeability, and light refractive properties5.
Vitamin E, a hydrophobic liquid that can block UV radiation, has been proposed as a diffusion barrier to prevent rapid drug diffusion¡Xprolonging drug release1,5-7. As vitamin E is biocompatible with the ocular surface, attaching a layer of vitamin E onto the surface of commercial contact lenses can increase the path length which drugs or other molecules are required to travel to diffuse out7. Sartini et al. demonstrated that contact lenses soaked in vitamin E barriers showed a drug release of around 3-fold compared to normal contact lenses6. In the study, contact lenses treated with vitamin E were able to release 676.7 £gg of dorzolamide and 191.4 £gg of timolol, whereas 217.8 £gg of dorzolamide and 60.6 £gg of timolol were released from untreated control6.
Nonetheless, further studies have to be done to look into the durability of the vitamin E barrier in contact lens storage and when the lenses are handled. Whether the barrier function remains effective after a thorough contact lens cleansing regimen or if they would be more suitable for disposal after one-time use.
Drug Reload in Imprinted Contact Lenses
A complimentary template can be created with high-affinity drug binding cavities during molecular imprinting process, allowing the imprinted contact lenses to be refuelled by soaking in drug solution (Figure 1b). High-affinity binding monomers in the lens can help to increase drug loading capacity1. However, to have a constant controlled release of active drug over time, the ratio of functional monomers to medication and the concentration of polymer cross-linker needs to be considered carefully1.
Previous trial on timolol delivery with rabbit model showed that molecularly imprinted contact lenses gave a drug release duration of 180 minutes; whilst soaked contact lenses provided a release span of 90 minutes and that of eye drops was 60 minutes. Further evaluation indicated that imprinted contact lenses had 3.3- and 8.7-fold bioavailability than soaked contact lenses and eye drops, respectively6.
Acrylic acid (AA), acrylamide (AM), methacrylic acid (MAA), methyl methacrylate (MMA) and N-vinyl 2-pyrrolidone (NVP) have been considered as possible high-affinity monomers5. However, most of these were tested in vitro or yet to be tested on human subjects. Therefore, further investigations would be required to ensure the sustained release of drug and which functional monomer is the most favourable.
Drug Implants in Contact Lenses
Desai et al. raised the idea of drug implants in the outer periphery of contact lenses (Figure 1c)6, which allows more than one drug to be implanted into the contact lens. Implants are entrapped into contact lenses. Active drug is loaded onto a ring film to make up implants. Maulvi et al. designed a frontally-located HA-loaded poly-HEMA implant onto the poly-HEMA lens matrix. The HA-loaded poly-HEMA implant was first synthesised by mixing HA with the HEMA/MAA monomer mixture and subsequently photo-polymerized. After placing the prepared implant in a female mould, excess pre-monomer mixture was added, and UV polymerisation was done by placing the male mould in position. As a result, the prepared implant was incorporated into poly-HEMA contact lenses at the front surficial region (Figure 2)8.
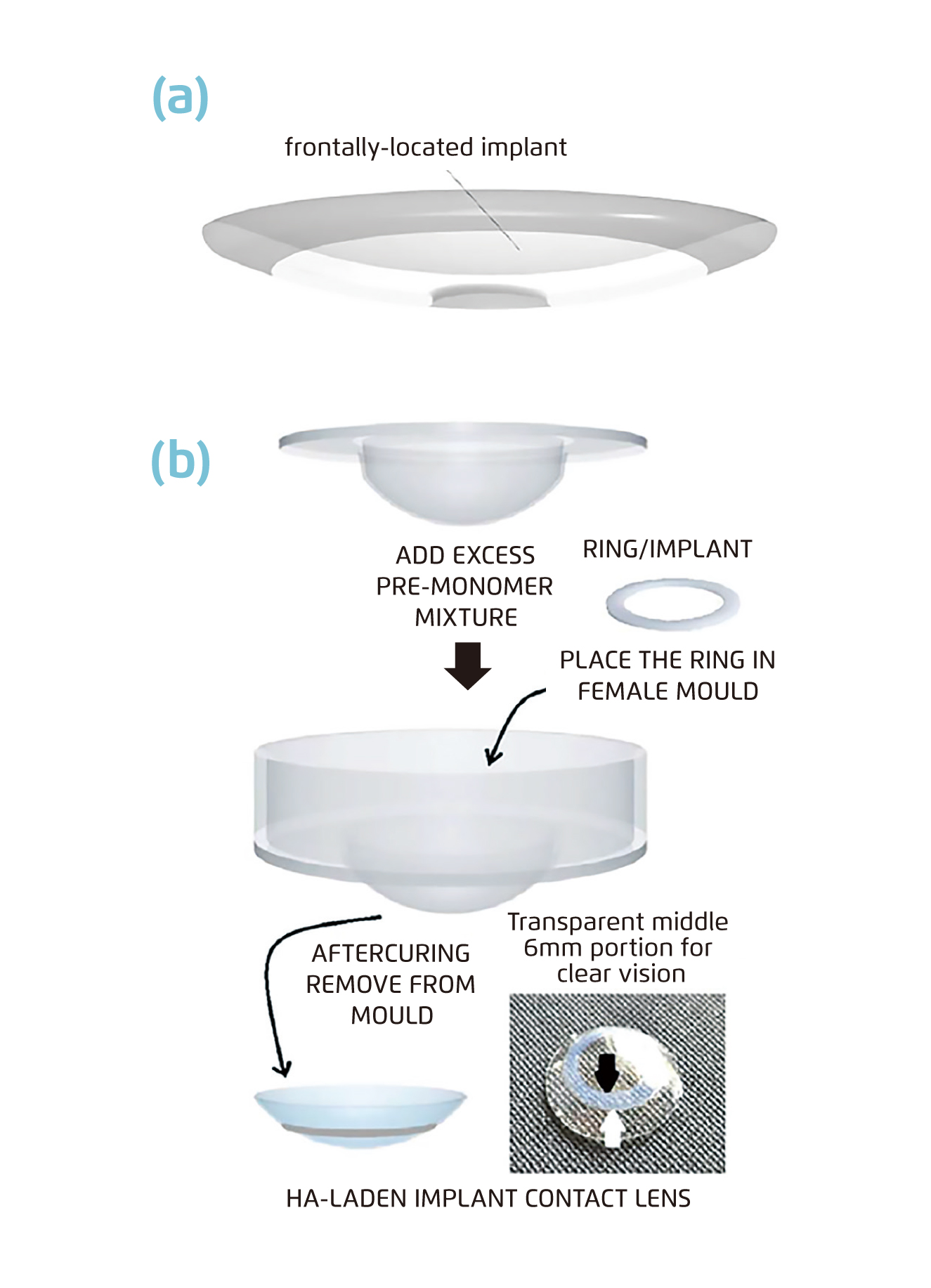
Figure 2.
Schematic of the fabricating process of contact lenses embedded with implant (Black arrow: inner margin of the implant, white arrow: outer margin of the implant) 8.
As the study shows, researchers loaded each implant with bimatoprost, timolol, or hyaluronic acid. Hyaluronic acid to improve comfort for prolonged duration of contact lens wearing. Soaked contact lenses (with timolol and bimatoprost) were compared with contact lenses with implants (timolol, bimatoprost and hyaluronic acid). Eye drops reduced intraocular pressure by 3.10 mmHg at maximum and returned to baseline after 12 hours. The soaked contact lenses and the contact lenses with implants lowered intraocular pressure by 4.33 mmHg and 5.00 mmHg and kept intraocular pressure below baseline values for 96 hours and 120 hours, respectively. A sustained drug release of 17.26 hours was found in tears with implanted contact lenses, in contrast with soaked contact lenses (6.61 hours) and eye drops (11.47 minutes)6. Similar results were found when using timolol alone.
Limitations to Overcome
Contact lenses as a novel pharmaceutical agent to aid in drug delivery has a bright future and a potential market. Commercially available contact lenses have already demonstrated in vitro results in releasing a significant amount of drug into the ocular system, though in an uncontrolled pathway. Therefore, the next step would be to look into tailoring controlled drug release kinetics and drug loading7. Moreover, further enhancement in visibility and comfort of the contact lenses would likely promote adherence and compliance to treatment, which hence improves patients’ quality of life. Besides, the issues of biocompatibility and sterilisation and preservation against microbes are noteworthy. The use of preservatives may present its challenge, as the lens itself is liable to take up and release these molecules, which may lead to undesirable amount of preservatives on the ocular surface. As well as reducing drug loading volume as preservatives may also take up partial capacity7.
With the current pace of ocular research and efforts being made and put in, it is expected to result in a contact lens formulation that retains high precorneal residence time, in turn increasing bioavailability, avoids non-specific drug tissue accumulation and delivers therapeutic drug levels into the targeted ocular tissue3.
References
1. Franco and De Marco. Polymers (Basel). 2021;13(7):1102. 2. Peral et al. Appl Sci. 2020;10(15). 3. Patel. World J Pharmacol. 2013;2(2):47. 4. Robin and Muir. Expert Rev Ophthalmol. 2019;14(4-5):199-210.
5. Filipe et al. Rev Bras Oftalmol. 2016;75(3):241-247. 6. Sartini et al. Appl Sci. 2021;11(2):724. 7. Hui. Clin Exp Optom. 2017;100(5):494-512. 8. Choi and Kim. Materials (Basel). 2018;11(7):1125.





