
Associate Consultant,
Department of Medicine,
Queen Mary Hospital,
University of Hong Kong
Pomalidomide in Lenalidomide-refractory Multiple Myeloma
The introduction of immunomodulatory drugs (IMiDs) as first-line therapy and after relapse has improved response rates and survival in patients with multiple myeloma (MM). However, the outcomes of MM are still hindered by the occurrence of refractory to therapies. Practically, a substantial proportion of patients becomes refractory to bortezomib and lenalidomide leaving limited treatment options for these patients. Hence, the development of pomalidomide-based regimens has brought new hope for patients with relapsed/refractory MM (RRMM). To uncover the practical issues in treating RRMM in local settings, Dr. Karen HK Tang of the Queen Mary Hospital shared the case of her patient with RRMM receiving IMiDs therapy.
Background Information
The patient of the current case was a 64-year-old male with documented ischemic heart disease and received 3 times of percutaneous coronary intervention (PCI), respectively in 2004, 2005 and 2013. In 2015, the patient complained on back pain. Blood tests indicated that his IgG level was 7110 mg/dL and serum protein electrophoresis (SPE) was 52 g/dL. Moreover, flow cytometry reflected 33% clonal plasma cells in peripheral blood while extensive infiltration of clonal abnormal looking plasma cells was observed in bone barrow. However, there was no high-risk cytogenetics observed. Besides, multiple osteolytic lesions with low FDG avidity was detected under PET/CT. Thus, the ISS stage 3 IgGl-lambda MM was diagnosed.
First-line Treatment and Initial Outcomes
To control the diagnosed MM, Dr. Tang prescribed bortezomib, thalidomide, and dexamethasone (VTD) for the patient in December 2015, then switched to bortezomib, cyclophosphamide and dexamethasone (VCd) due to constipation and severe malaise experienced by the patient after thalidomide. After 5 cycles of VCd, the value of SPE was reduced to 5.5 g/dL.
Peripheral blood stem cell (PBSC) was harvested in June 2016. Dr. Tang decided to prescribe 4 cycles of VRd before autologous stem cell transplantation (ASCT) because of the suboptimal response. Then, the patient achieved very good partial response (VGPR) with SPE reduced to 1.9 g/dL in September 2016. Hence, ASCT was performed in November 2016 and the patient achieved complete response (CR) after ASCT. PET/CT showed improvement in all previous osteolytic lesions. Thus, the patient started the lenalidomide maintenance therapy after ASCT.
Development of Relapsed Disease
In April 2018, an increase in SPE to 1.9 g/dL was noted, and dense focal infiltrates of plasma cells were observed in bone marrow. Moreover, subsequent PET/CT showed reappearance of multiple osteolytic lesions with definite FDG uptake. The SPE increased to 23 g/dL in October 2018.
Pomalidomide-based Therapy for Lenalidomide-refractory MM
To tackle the relapsed disease, Dr. Tang prescribed daratumumab plus pomalidomide with low-dose dexamethasone (Dara-Pom-d, pomalidomide dosage: 4 mg, 28-day cycle) in November 2018. Impressively, the SPE dropped rapidly to 9 g/dL within 1 month upon the pomalidomide-based treatment. Then, VGPR was achieved in April 2019 and CR was subsequently achieved in July 2019. Moreover, MRD-negative at 0.8 x 10-5 using LymphoTrack®-Miseq platform was achieved as well.
Of note, during Dara-Pom-d treatment, the patient was complicated with suspected fungal pneumonia in June 2019 that a cavitory lesion sized 5 x 4 x 3 cm with internal fluids and gas density in the right upper lobe (RUL) was identified by thorax computed tomography (CT, Figure 1). The patient was complicated by pulmonary embolism (PE) as well. Thus, voriconazole and edoxaban were prescribed while pomalidomide was temporarily withheld for 2 months.
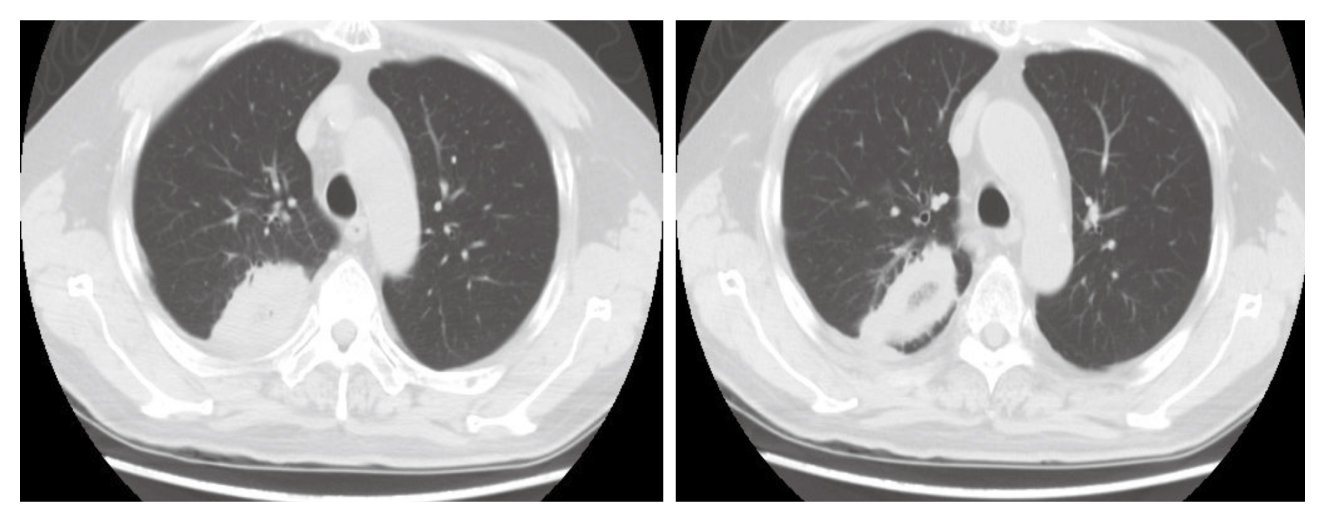
Figure 1. Cavitory lesion with internal fluids and gas density in RUL
In September 2019, pomalidomide treatment was resumed, but daratumumab and dexamethasone were stopped. After 6 months of edoxaban, the treatment was switched back to aspirin. CR was achieved in September 2019 and remained till September 2020, thought a rise in SPE to 2.97 g/dL was observed in November 2020. The patient was satisfied with the pomalidomide treatment. Thus, prescription of full-dose pomalidomide was continued upon the patient’s request. Besides, the 3 weekly treatments of daratumumab were resumed. Since then, the SPE was remained at 2 g/dL with no evidence of progression. A summary of the level of SPE throughout the treatment course was illustrated in Figure 2.
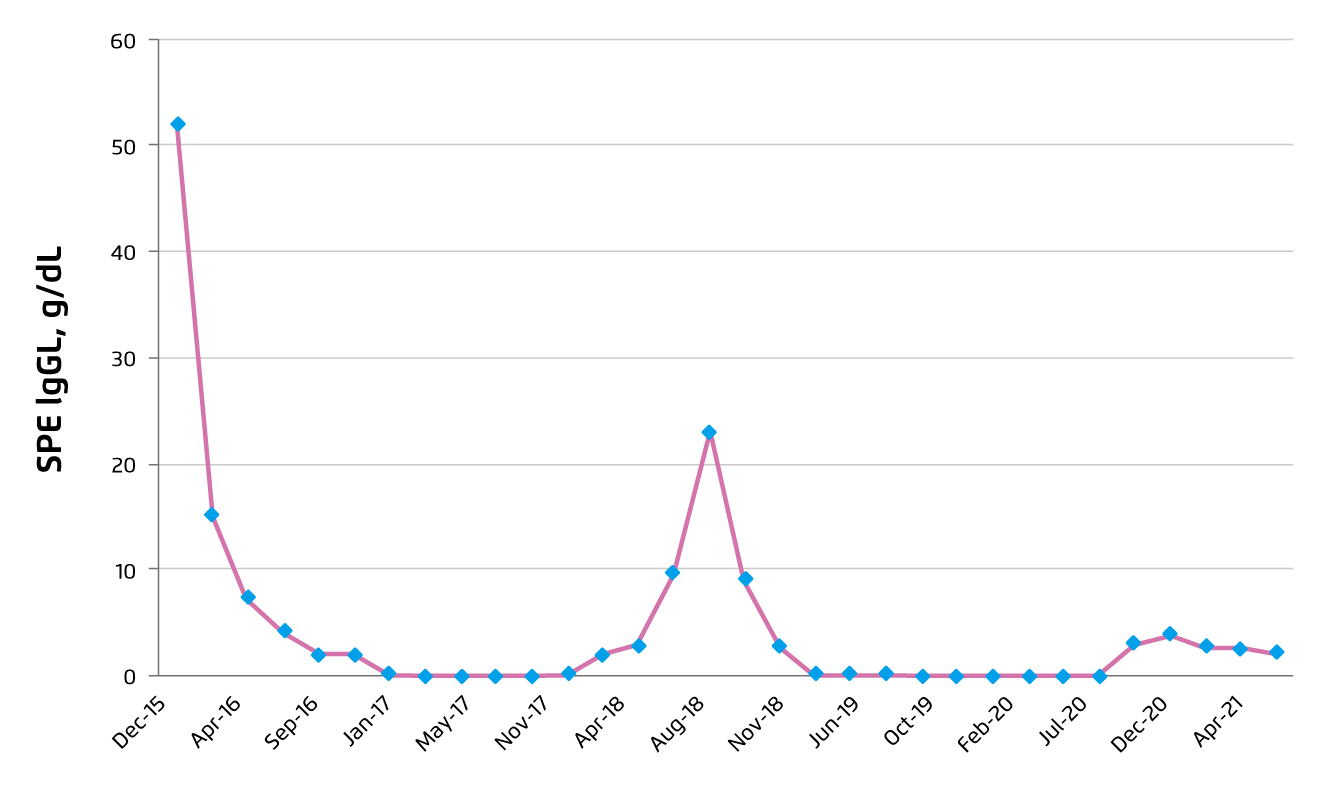
Figure 2. Summary on SPE level during treatment course
Countering Lenalidomide-refractory MM
By definition, patients with lenalidomide-refractory MM would have no response, i.e. less than partial response, while receiving lenalidomide-containing treatment or progression on or within 60 days of a lenalidomide-containing regimen1. As one of the main treatments recommended for lenalidomide-refractory MM, pomalidomide exerts its tumouricidal and immune stimulating effects through binding to cereblon. Essentially, although the exact mechanism of how pomalidomide overcome lenalidomide refractoriness is still unknown, Dr. Tang outlined that the effects of pomalidomide are distinct from lenalidomide, in terms of binding affinity to cereblon, substrate degradation kinetics and gene modulation profile2.
Dr. Tang commented that the proportion of lenalidomide-refractory individuals in some of the previous clinical trials such as ENDEAVOR study were low, ranging between 24-36%, so that the outcomes in these patients were under-represented3. In contrast, there are trials evaluated efficacy of pomalidomide-based regimens involving substantial proportions of lenalidomide-refractory individuals (≥70%). For instance, 89% of individuals in the EQUULEUS trial were lenalidomide-refractory. The results of EQUULEUS trial demonstrated that Dara-Pom-d treatment yielded a median progression-free survival (PFS) of 8.8 months and the median overall survival (OS) was 17.5 months (Figure 3). 42% of patients achieved VGPR or better4.
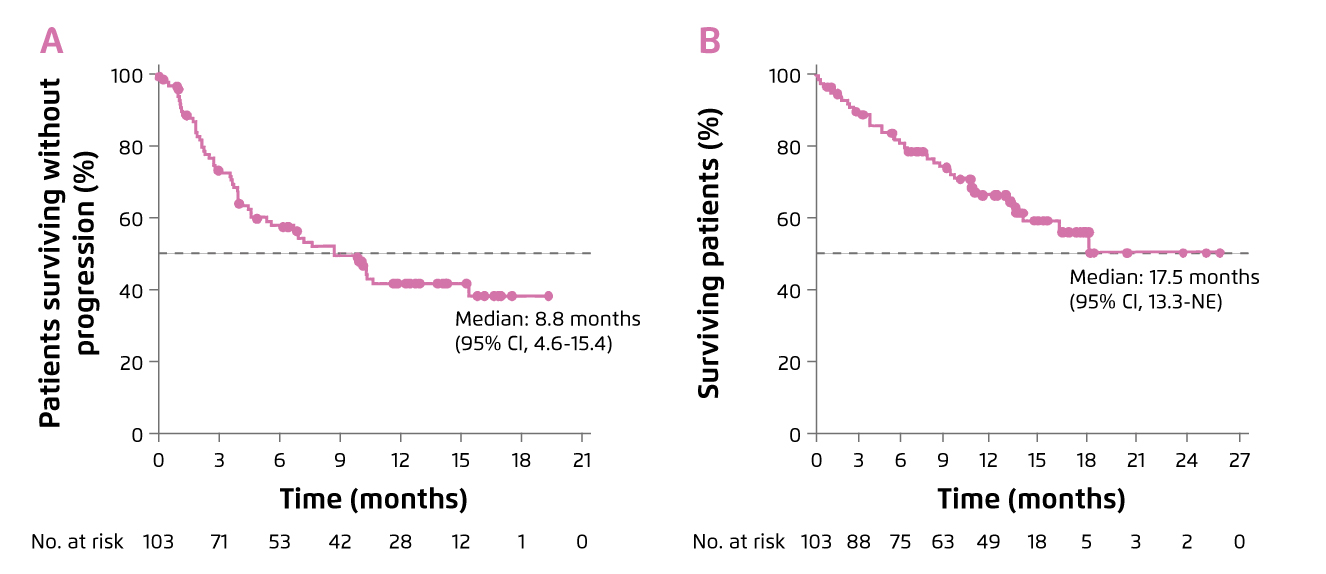
Figure 3. Survival benefits achieved by Dara-Pom-d treatment, A) PFS and B) OS4
Dr. Tang addressed that CD38 is expressed at high levels on myeloma cells and on immune effector cells, including natural killer (NK) cells, which are important for daratumumab-mediated antibody-dependent cellular cytotoxicity (ADCC)5. Of note, pomalidomide has been shown to upregulate CD38 in MM cells to enhance daratumumab-mediated ADCC6.
In the current case, MRD-negative status was achieved in this lenalidomide-refractory patient by the salvage Dara-Pom-d treatment, and the CR was sustained for 1.5 years. Dr. Tang emphasised that pomalidomide is best studied for lenalidomide-refractory MM. She concluded that, for lenalidomide-refractory MM, addition of cyclophosphamide to Pom-d may be beneficial in a resource restricted setting, whereas combined treatment of Dara-Pom-d is a reasonable option with favourable PFS.
References
1. Kumar et al. Leukemia 2012; 26: 149-57. 2. Bjorklund et al. Blood Cancer J 2015 510 2015; 5: e354-e354. 3. Dimopoulos et al. Lancet Oncol 2017; 18: 1327-37. 4. Chari et al. Blood 2017; 130: 974. 5. Casneuf et al. Blood Adv 2017; 1: 2105-14. 6. Boxhammer et al. J Clin Oncol 2015; 33: 8588.





