
The Potential and Challenge
of Gene-edited Cellular Therapies
The recent advances in genome editing allow precise manipulation of cellular DNA sequences and have raised hopes of curing serious genetic diseases. CRISPR-Cas9 is a specific and efficient gene-editing biotechnology that is massively employed for the development of next-generation cellular therapy. In recent years, CRISPR-edited cellular therapies have applied in humans and entered the early phase of clinical trials. In this article, the effectiveness and safety profile of gene-based medicine are briefly reviewed.
Therapeutic Application in Haemoglobin Production Defects
Sickle Cell Disease (SCD) and beta-thalassemia are monogenic disorders related to defective β-globin production. Patients with these diseases suffer from potential life-threatening manifestations, such as stroke or vaso-occlusive crises (VOC)1. Recently, the use of CRISPR gene-editing to increase the expression of foetal haemoglobin (Hbf) for treating SCD and transfusion-dependent β-thalassemia (TDT) has been evaluated in a phase II study. First, patients’ CD34+ haematopoietic stem and progenitor cells were isolated, and the BCL11A erythroid-specific enhancer was modified by CRISPR-Cas9, which boost the production of Hbf in erythroid cells. The edited autologous stem cells (CTX001) were then re-infused back to patients for therapeutic use (Figure 1)2.
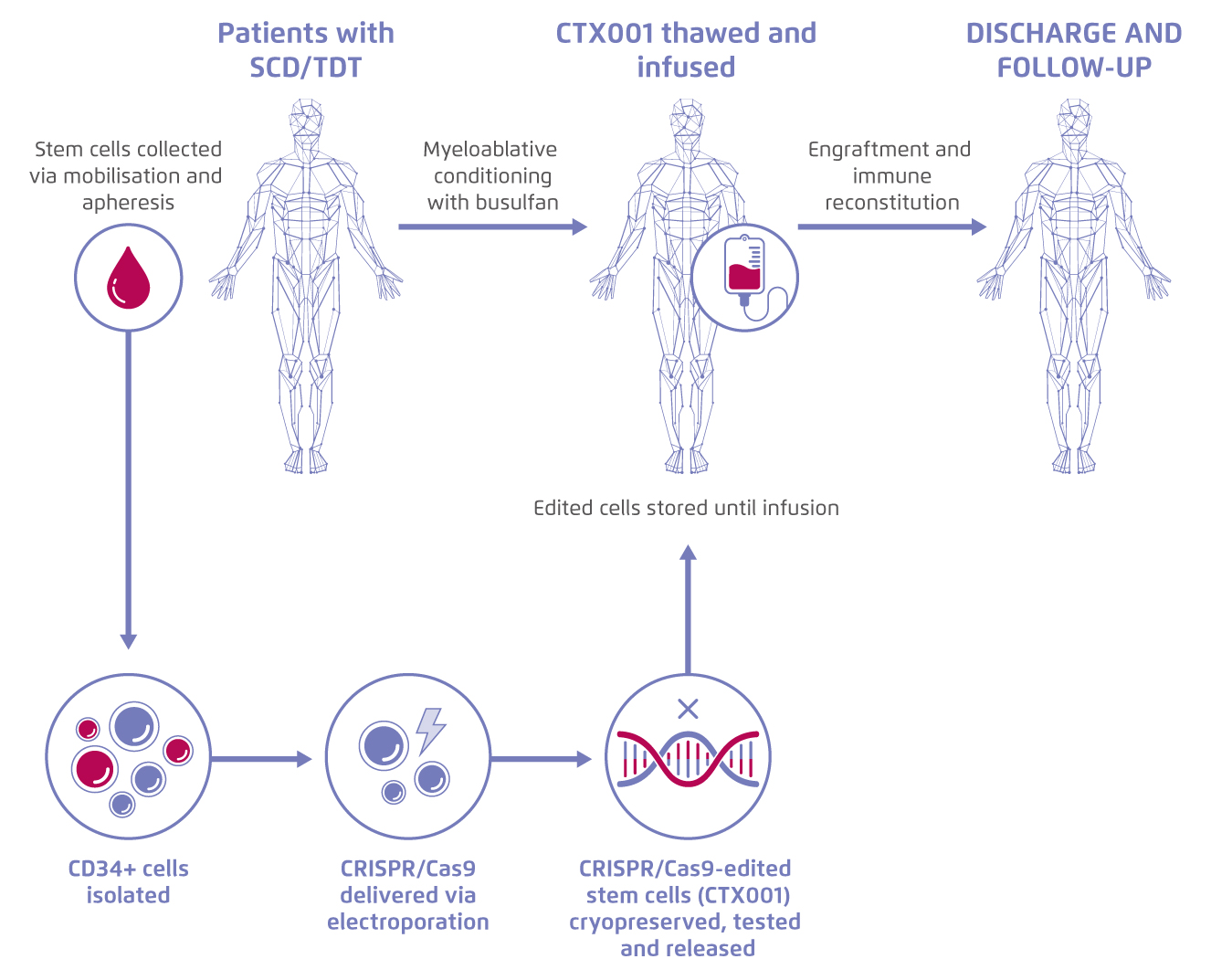
Figure 1.
Process of treatment with gene-edited autologous haematopoietic stem and progenitor cells. Figure adapted from CRISPR Therapeutics5. SCD: Sickle cell disease. TDT: transfusion-dependent β- thalassemia
The study showed that, with up to 22 months follow-up, levels of haemoglobin (Hb) and Hbf have been successfully increased in all TDT patients (n=7) over time3. The median Hbf was raised from 0.3 g/dL at baseline to 13.1 g/dL after 18 months of treatment, which accounted for 92.9% of total Hb. Patients stopped receiving packed red blood cells (pRBC) transfusion at 0.7-2 months, and notably, the first patient who received CTX001 has remained transfusion-free for over 20 months3.
Moreover, patients with SCD (n=3) have had no VOC after CTX001 infusion and the first patient has remained VOC-free for 16 months. Regarding the safety profile, adverse events (AEs) were generally consistent with myeloablation and autologous stem cell transplant. One patient experienced serious AEs that were possibly related to CTX001, including headache, haemophagocytic lymphohistiocytosis, acute respiratory distress syndrome, and idiopathic pneumonia syndrome, but all AEs were resolved later. These data suggested that CTX001 might be a potential functional cure for TDT and SCD.
Allogeneic CAR-T Therapy
Over several decades, the treatment of leukaemia/lymphoma was dominated by chemotherapy, radiation therapy, and stem cell transplantation. Recently, pharmaceutical companies and scientists developed chimeric antigen receptor T-cell (CAR-T) therapy based on the genetic and molecular interactions between cancer cells and the immune system. Comparing with autologous CAR-T (T-cells isolated from patients), the allogeneic approach (T-cells from healthy donors) allows flexible dosing, immediate availability, and better product consistency. However, allogeneic CAT-T may lead to life-threatening graft-versus-host disease (GvHD) and be eliminated by the host immune system4.
CTX110, an allogeneic CD19-directed CAR-T, is genetically edited to eliminate major histocompatibility complex I (MHC I) expression and prevent GvHD via T-cell receptor disruption3. A recent phase I, open-label study has evaluated the safety and response rate of CTX110 in patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) who had received at least two prior lines of therapy3. Patients (n=12) were infused with CTX110 following three days of lymphodepletion using fludarabine (30 mg/m2/day) and cyclophosphamide (500 mg/m2/day)3. With at least one month of follow-up, complete response (CR) was achieved in four patients and CAR-T was detected in the peripheral blood at multiple time points in eight patients, with peak expansion occurring at 1-2 weeks and cells detected as late as 180 days post-infusion3.
Importantly, the data revealed that allogeneic CAR-T therapy has an acceptable safety profile. No cases of GvHD, infusion reactions, and dose-limiting toxicities were reported in patients receiving low to moderate doses of CTX110 (30-300 x106 cells). Besides, cytokine release syndromes (≤grade 2) occurred in three patients and were resolved with tocilizumab administration. Immune effector cell-associated neurotoxicity syndrome (ICANS, grade 2) was also reported in one patient while improved within 24 hours with standard interventions. Periorbital cellulitis and febrile neutropenia appeared after CTX110 infusion, both of which were resolved and confirmed unrelated to disease progression or CTX1103.
Further Evidence is Required
Gene-edited cellular therapies are changing the therapeutic landscape of severe haematological diseases. The use of engineered haematopoietic stem cells with increased expression of foetal haemoglobin brings meaningful improvement to patients’ quality of life. The CRISPR-edited T-cells control the risk of life-threatening GvHD and increase accessibility to CAR-T therapy. However, as these data were derived from the early phase of clinical studies, longer observation is needed to validate their efficacy and safety.
References:
1. Dong AC & Rivella S. Adv Exp Med Biol. 2017;1013:155-176. 2. Frangoul H, et al. Safety and Efficacy of CTX001 in Patients with Transfusion-Dependent £]- thalassemia and Sickle Cell Disease: Early Results from the Climb THAL-111 and Climb SCD-121 Studies of Autologous CRISPR-CAS9-Modified CD34+ Hematopoietc Stem and Progenitor Cells. Poster presented at the 62nd ASN Annual Meeting and Exposition. 3. CRISPR Therapeutics. Creating transformative gene-bases medicines for serious diseases. Available at: https://crisprtx.gcs-web.com/static-files/8940034e-9f73-47df-91b3-93e77c0a2a53 (Accessed on 20 Mar 2021) 4. Depil S, et al. Nat Rev Drug Discov. 2020;19:185-199. 5. CRISPR Therapeutics. Hemoglobinopathies. Available at: http://www.crisprtx.com/programs/hemoglobinopathies (Accessed on 20 Mar 2021)





