Nutrition Intervention for Improving Health Outcomes of Cancer Patients
Malnutrition is prevalent among cancer patients, whereas up to 70% of patients are at risk. The condition can adversely affect treatment outcomes, quality of life and survival1. While many patients are already malnourished upon diagnosis of cancer, malnutrition can develop over the course of cancer treatment period and frequently underdiagnosed and undertreated. Essentially, malnutrition is associated with adverse health outcomes in patients with cancer, such as increased mortality and lengthened duration of hospital stay2. Thus, adequate nutritional intervention during and after cancer therapy is vital for optimising overall treatment outcomes as well as improving quality of life of cancer patients.
Malnutrition in Cancer Patients
Malnutrition is common among patients with cancer, with reported prevalence ranges between 40 and 80% depending on the type and location of tumour, stage of disease, treatment received as well as the method of nutritional assessment applied3. Cancer-related malnutrition is multifactorial that reduced dietary intake, cancer cachexia, and nutrition impact symptoms may all contribute to the condition. Of importance, the side effects of cancer treatments including chemotherapy and radiotherapy are reported to produce symptoms, such as anorexia, nausea, vomiting, and diarrhoea, leading to loss of appetite and hence adversely impact on patient’s nutritional status4. Moreover, acute and chronic inflammation are important etiologic factors in the pathogenesis of malnutrition due to associated metabolic alterations and anorexia5.
Protein-energy malnutrition (PEM) is a nutritional status in which reduced availability of nutrients leads to changes in body composition and function. In addition to loss of appetite, PEM in cancer patients can be caused by physical inability to ingest food and metabolic alterations including insulin resistance, glucose intolerance, energy imbalance and increased lipolysis and proteolysis6. If PEM is left untreated, it may progress to severe wasting associated with cancer anorexia-cachexia syndrome, a major paraneoplastic syndrome characterised by metabolic abnormalities and loss of skeletal muscles with or without loss of adipose tissues6.
Unlike starvation and simple malnutrition, cancer-associated cachexia can only be partially but not entirely reversed by conventional nutritional support. The possible consequences of cancer-associated cachexia include increased chemotherapy toxicity, complications from cancer surgery and mortality. Notably, cancer-associated cachexia is frequently associated with pancreatic, oesophageal, gastric, pulmonary, hepatic, and colorectal cancers (Figure 1), probably due to their direct effects on ingestion, digestion and absorption of nutrients and/or their diagnosis at advanced stage7. In addition to altered energy balance described above, pro-cachexia cytokines and factors secreted by tumours directly elicit catabolism in target tissues are potential mediators of cachexia8. Moreover, endocrine, metabolic and central nervous system perturbations have also been reported to combine with the mediators to elicit catabolic changes in skeletal and cardiac muscle and adipose tissue7. To date, effective treatment completely reverses cancer-associated cachexia is yet to be available. Thus, screening for early recognition and timely nutritional support are crucial for preventing malnutrition and related complications in cancer patients.
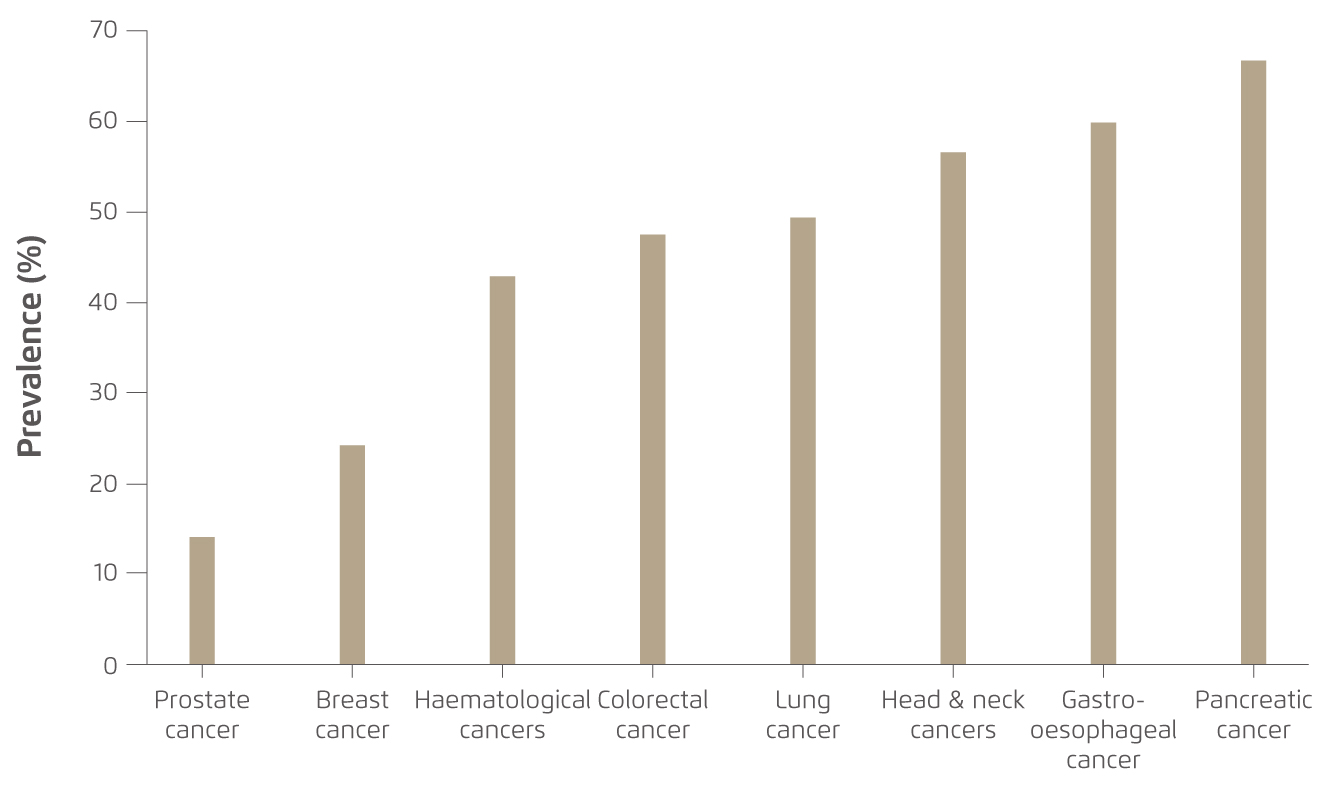
Figure 1. Cancer-associated cachexia by tumour site7
Nutritional Status Predicts Health Outcomes
Undoubtedly, the stage of cancer would influence the overall health outcomes of patients. Nonetheless, the interaction between nutritional status, disease/symptoms and therapeutic intervention would determine patient’s quality of life. For instance, previous cross-sectional study involving patients with cancers in head and neck or gastrointestinal (GI) tract indicated that significant reduction in energy and protein intake was observed in patients with stage III/IV disease as compared to their usual intake (p=0.001, Figure 2) and the intake of patients with stage I/II disease (p=0.0002). The results further revealed that the quality of life of patients, as represented by the quality of life function score, was determined by cancer location (effect size: 30%, p=0.0001), nutritional intake (effect size: 10%, p=0.01), weight loss (effect size: 30%, p=0.0001) and treatment prescribed such as chemotherapy (effect size: 10%, p=0.001) and surgery (effect size: 6%, p=0.01)9. The findings highlighted that, in addition to the stage and location of cancer, deterioration in nutritional status and reduction in nutritional intake would negatively impact the quality of life of patients, particularly among those with advanced diseases.

Figure 2.
(A) Energy and (B) Protein intake in cancer patients9, HN: head-neck, OES: oesophagus, STO: stomach, CR: colorectal
Besides quality of life, malnutrition is a marker of adverse outcomes in cancer patients. Sungurtekin et al (2004) demonstrated that, among cancer patients received major intraabdominal surgery, higher death rate and risk of complications were observed in malnourished patients10. On the other hand, chemotherapy can be associated with severe toxicity leading to dose delays, dose reductions, and treatment termination, known as dose-limiting toxicities (DLTs). While malnutrition would reduce lean mass, it has been reported that low lean mass was associated with increased toxicity to chemotherapy in both early- and late-stage disease regardless of cancer site and type of systemic chemotherapy. One of the possible reasons for the increased toxicity in patients with low lean mass is the alteration in the distribution, metabolism, and clearance of systemic chemotherapy drugs11.
In contrast to malnutrition, a recent systemic review by Richards et al (2020) summarised that early nutrition interventions, including nutrition counseling, oral nutrition supplements, or combination of both, were found to improve body weight and body mass index (BMI), nutrition status, protein and energy intake, quality of life, and response to cancer treatments1. This further supports the determining role of nutritional status in optimising health outcomes for cancer patients and the importance of timely nutritional intervention.
Nutritional Intervention for Cancer Survivors
Majority of literature advocates nutritional intervention would improve health outcomes in cancer patients and integration of it in cancer care was recommended. Practically, early detection of potential malnutrition is essential, whereas nutritional screening should be instituted by all members of the healthcare team caring the cancer patient, including physicians, nurses, dietitians, social workers and psychologists, since multidisciplinary variables (e.g. food intake, physical performance, psychological, financial cost and social functioning) have to be considered for each patient12.
Currently, there are various tools for assessing nutritional status of cancer patients. For instance, the Patient-Generated Subjective Global Assessment (PG-SGA) is a simple and inexpensive approach to identifying individuals at nutritional risk. The PG-SGA facilitates evaluation on the patient’s weight history, food intake, symptoms, and function13. Of note, recent opinion suggested the body composition assessment (BCA) to be determinant for interventions, treatments and outcomes14. Bioelectrical impedance can be applied to estimate body composition. With estimation of lean body mass, estimates of resting energy expenditure can be made13.
Nutritional intervention has to be individualised. Nonetheless, the first rule of nutrition intervention is to use the gut when it is available, and the preferred method of nutrition support is via the oral route with food. Appetite stimulants may be used to enhance appetite and to facilitate weight gain in the presence of significant anorexia. Moreover, supplemental enteral nutrition in the form of protein powders between meals are indicated when the GI tract is functional, but oral intake may be insufficient to meet nutritional needs15. Of importance, the recommendations by registered dietitians in dietary modifications are crucial.
A major goal of nutritional intervention for cancer patients is to favourably influence body composition, with the potential to improve cancer therapy outcomes, morbidities and ultimately, prognosis. To achieve this, individualised counselling based on comprehensive assessment of clinical and nutritional parameters is needed. Several guidelines to date include nutritional counselling as the standard of care for malnourished patients or at risk of malnutrition16.
In summary, nutritional status has a strong impact on the disease prognosis and quality of life for cancer patients. Nutritional screening in cancer patients allows for the early detection of malnourished patients and makes prompt nutritional intervention possible, which hence preventing nutritional deterioration and muscle wasting. Adequate and effective nutritional intervention during the disease course and treatment is essential for producing the best impact on patients’ outcomes.
References:
1. Richards et al. Nutrients.2020; 12: 1-19. 2. Pressoir et al. Br J Cancer 2010; 102: 966-71. 3. Tong et al. Support Care Cancer 2009; 17: 83-90. 4. Lis et al. Nutr. J.2012; 11. DOI:10.1186/1475-2891-11-27. 5. Jensen et al. J Parenter Enter Nutr 2013; 37: 802-7. 6. Gangadharan et al. Oncotarget.2017; 8: 24009-30. 7. Baracos et al. Nat Publ Gr 2018; 4. nrdp.2017.105. 8. Fearon et al. Cell Metab.2012; 16: 153-66. 9. Ravasco et al. Support Care Cancer 2004; 12: 246-52. 10. Sungurtekin et al. J Am Coll Nutr 2004; 23: 227-32. 11. Ryan et al. Nutrition 2019; 67-68. j.nut.2019.06.020. 12. Tchekmedyian et al. JPEN J Parenter Enteral Nutr, 1992. DOI:10.1177/014860719201600610. 13. Bauer et al. Eur J Clin Nutr 2002; 56: 779-85. 14. Ravasco et al. J Clin Med 2019; 8: 1211. 15. Heber et al. Med. Clin. North Am.2016; 100: 1329-40. 16. Arends et al. Clin Nutr 2017; 36: 11-48.