
Relieving the Health Burden of Metastatic Castration-Resistant Prostate Cancer
Prostate cancer is considered one of the most prevalent cancers in men worldwide. In Hong Kong, it is the fourth leading cause of male cancer deaths, with both age-standardised incidence and mortality rates rising since the 1980s1. Particularly, around 16% of patients with prostate cancer are metastatic castration-resistant (mCRPC) at the time of diagnosis2, which is almost unresponsive to traditional hormone therapies. Nonetheless, recent clinical trials demonstrated that poly(ADP-ribose) polymerase (PARP) inhibitors can significantly improve the health outcomes of mCRPC.
Current Treatments and Challenges in mCRPC
While the 5-year survival rate for local early-stage prostate cancer can be well above 90%, in its metastatic form this rate drops drastically to just 31%3, with expected survival of fewer than 2 years from initial progression4. The current first-line treatments aim to lower the number of circulating androgens and decrease overall androgen signalling5. This is commonly known as Androgen Deprivation Therapy (ADT). However, the effectiveness of ADT is often hindered by the development of resistance and subsequent progression to mCRPC6.
As resistance develops, patients often proceed to second-line chemotherapies or immunotherapies to extend survival. However, the heterogeneity of prostate cancer progression poses another major challenge in deciding appropriate therapies. Treatment regimens for mCRPC can be highly variable depending on the stage and progression of disease. For instance, chemotherapeutic agents like docetaxel (DTX) impede cancer cell mitosis to cause apoptosis2, whereas sipuleucel-T vaccine targets and upregulates patients’ T-cell cytotoxicity against cancer cells7. Not only are the efficacies of these therapies highly variable, their associated adverse events gain clinical concern as well2,7. Thus, a definitive therapy that is effective in slowing disease progression and improving overall prognosis is highly desirable for patients with mCRPC.
An Overview on PARP Inhibition and Rucaparib
DNA damage repair (DDR) pathways are novel targets for cancer treatments. Inherited and acquired mutations in DDR genes contribute significantly to the development of malignant tumours8, as in the case of mCRPC. Poly(ADP-ribose) polymerase (PARP) is an enzyme responsible for repairing single-strand breaks (SSBs)9. The primary goal of PARP inhibitor therapy is to inhibit SSB repair in cancer cells containing defective DDR genes. The presence of unrepaired SSBs will then evolve into DNA double-strand breaks (DSBs) in the cell. Since these cells are incapable of carrying out homologous recombination, broken DNA ultimately leads to cancer cell death in a process known as synthetic lethality (Figure 1)10.
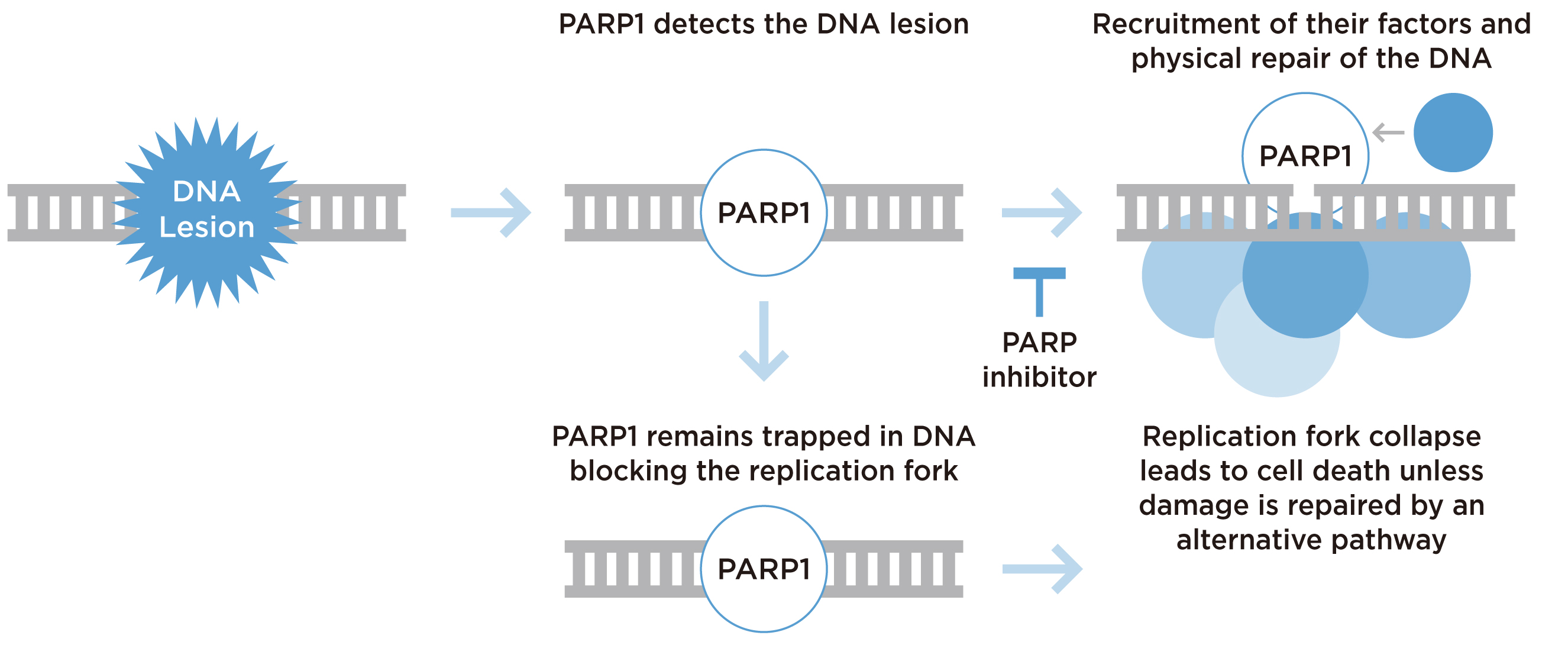
Figure 1. Mechanism of PARP inhibition10
Rucaparib is a PARP inhibitor previously approved as a monotherapy for treating advanced and progression ovarian cancer11. Thanks to the better understanding in prostate cancer genomics, rucaparib has begun expanding its indication into mCRPC. A genomic study by Robinson et al. (2015) identified DDR gene alterations in approximately 23% of mCRPC patients, with BRCA2 being the most frequent one12. Hence, this highlights the potential efficacy of rucaparib in mCRPC patients with BRCA gene mutations.
Clinical Efficacy of Rucaparib in mCRPC
The clinical benefits of rucaparib in mCRPC were demonstrated in the TRITON2 trial, a multicentre, open-label phase II study, which included 190 mCRPC patients with various DDR gene alterations who were unresponsive to ADT and taxane-based therapies. The results demonstrated that rucaparib yielded an objective response rate (ORR) of 43.9% (95% CI: 30.7%-57.6%) in patients with BRCA1/2 alterations, and majority of responses lasted for more than 24 weeks. Furthermore, rucaparib was shown to produce a prostate-specific antigen (PSA) response of 52% (95% CI: 41.7%-62.2%) in patients with BRCA1/2 alterations (Figure 2)13. Essentially, rucaparib exhibited superior efficacy and anti-tumour activity as compared with the reported efficacies of other therapies including abiraterone acetate, which PSA response was 29%14, and sipuleucel-T, which PSA response was 4.8%15. The results further reported that rucaparib was well-tolerated and manageable, with the most common treatment-emergent adverse events (TEAEs) being fatigue, nausea, and anaemia13. However, the therapeutic benefits of rucaparib appeared to be limited mainly to BRCA-associated mCRPC, as objective responses were not observed in patients carrying other DDR gene alterations, such as ATM or CDK128. Nonetheless, due to the promising efficacies demonstrated in the TRITON2 trial, rucaparib was recently granted accelerated approval by the FDA for patients with deleterious BRCA mutation-associated mCRPC16.
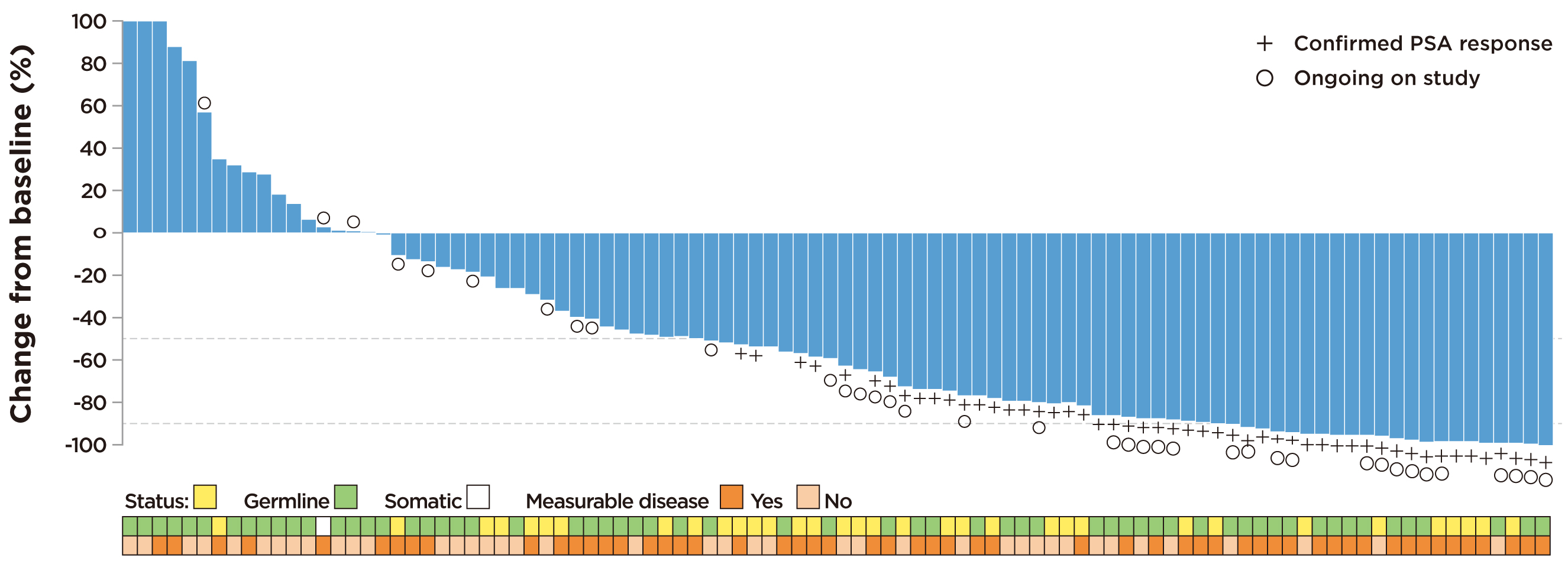
Figure 2. Observed change from baseline in PSA in rucaparib-treated patients with BRCA1/2 alteration13
Identifying the Suitable Patients
While the treatment outcome of mCRPC with conventional therapies is suboptimal, clinical trial results demonstrated the improved survival benefits achieved by rucaparib. Of importance, previous report suggested the particularly strong specificity towards mCRPC patients harbouring deleterious BRCA mutations. This therefore highlights the importance of future germline and somatic testing for patients with high-risk for metastatic prostate cancer, so as to identify individuals most suitable for PARP inhibitor therapies. Last but not least, further evaluation on the application of rucaparib in earlier lines of mCRPC treatment and its potential efficacies in combination with existing therapies are required for further optimisation of treatment outcomes for patients with mCRPC.
Acknowledgement
The editorial support by Mr Gavin Chiu of the School of Biomedical Sciences, CUHK in the preparation of this article is deeply appreciated.
References
1. Centre for Health Protection. Prostate Cancer. 2020. 2. Sathianathen et al. Cochrane Database Syst. Rev. 2018. 3. American Society of Clinical Oncology. Prostate Cancer. 2020. 4. Frieling et al. Cancer Control 2015; 22: 109-20. 5. Ahmed et al. J Cell Physiol 2014; 229: 271-6. 6. Teo et al. Annu Rev Med 2019; 70: 479-99. 7. Anassi et al. PT 2011; 36: 197-202. 8. Ratta et al. Prostate Cancer Prostatic Dis. 2020. 9. Adashek et al. Cells. 2019; 8. 10. Virtanen et al. Genes (Basel). 2019; 10. 11. Pearre et al. Ther Clin Risk Manag 2018; Volume 14: 2189-201. 12. Robinson et al. Cell 2015; 161: 1215-28. 13. Abida et al. Ann Oncol 2019; 30: v327-8. 14. De Bono et al. N Engl J Med 2011; 364: 1995-2005. 15. Garcia et al. Ther. Adv. Med. Oncol. 2011; 3: 101-8. 16. FDA. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. 2020





