A Beacon of Hope for Patients with Metastatic Triple Negative Breast Cancer (mTNBC)
Frequently afflicting young women below 50 years old, Triple Negative Breast Cancer (TNBC) accounts for 14% of breast cancer cases in Hong Kong1. Classified by the lack of expression of estrogen and progesterone receptors and human epidermal growth factor receptor-2c protein which restricts the use and development of targeted therapies, TNBC is more aggressive and has a higher risk of metastasis compared to other breast cancer subtypes2. Whilst chemoresistance is common after first-line chemotherapy, the health outcomes of the current standard of care are suboptimal3 and the reported median survival of patients with metastastic TNBC (mTNBC) is less than one year4. Although innovative treatments such as immune checkpoint inhibitors are generally safe and tolerable, the response rate and clinical benefit are yet to be satisfied in certain cases5. Nonetheless, the recent approval of Sacituzumab Govitecan-Hziy (SG) sheds light on innovative and revolutionary treatments for mTNBC.
Structural and Functional Features of SG
While sharing some common structural features with other second-generation antibody-drug conjugates (ADG), SG also has a number of unique characteristics. It is composed of three components: a monoclonal antibody hRS7 IgG1κ against trophoblast cell surface antigen 2 (Trop-2), SN-38 which is a semi-synthetic camptothecin and a CL2A linker (Figure 1)6. Increased expression of Trop-2 confers selective advantage and stimulates cell transformation and proliferation, which is associated with higher risk of metastasis and shorter survival7.
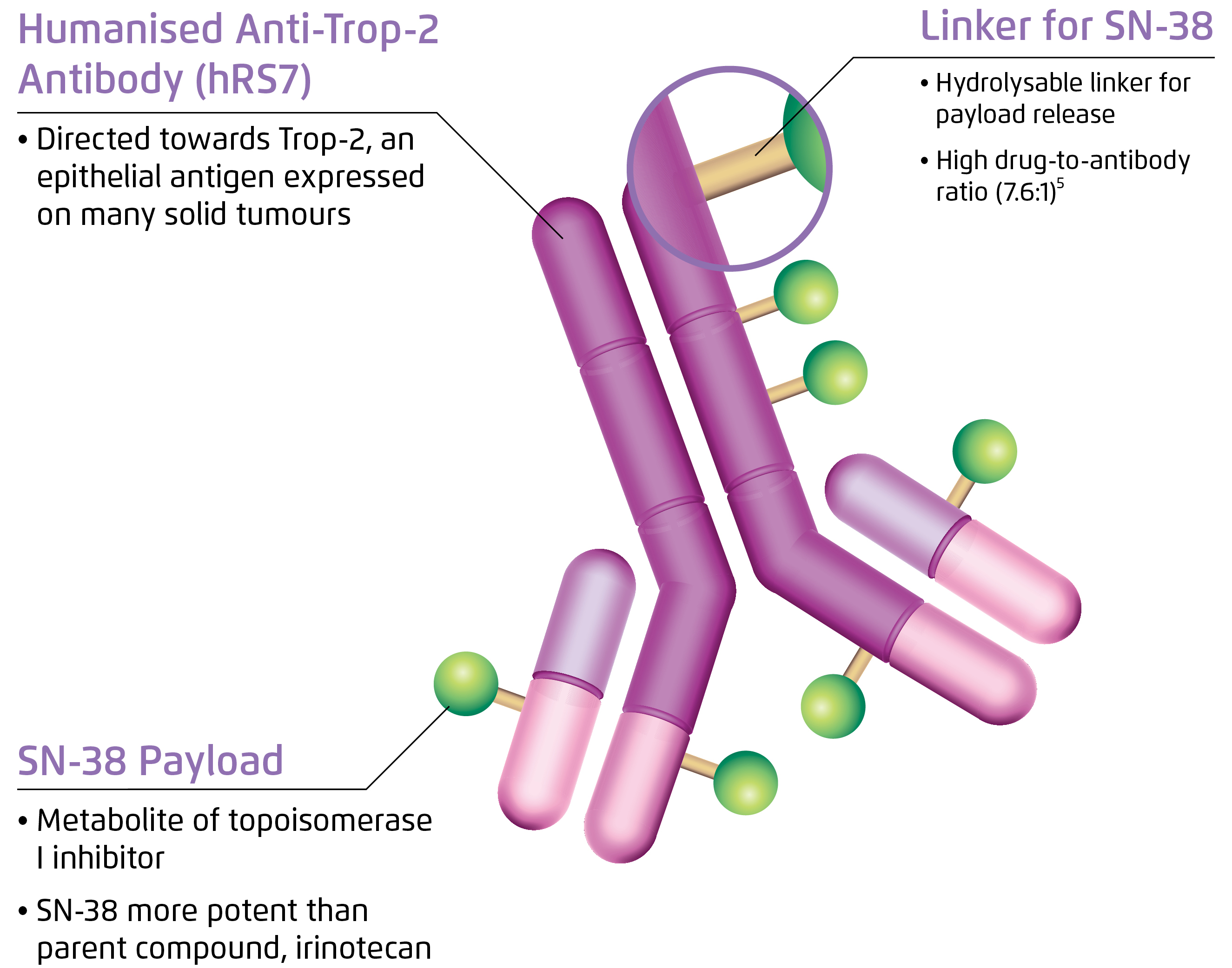
Figure 1. Structure of SG6
Besides, camptothecin is an active metabolite of irinotecan exerting cytotoxicity by binding to and stabilising nuclear topoisomerase I (TOPOI)/DNA covalent complex, blocking replication fork and inducing double strand break during S phase and ultimately causing cell death by abrogating DNA, RNA and protein synthesis8. Notably, SG has been shown to promote apoptosis by enhancing the cleavage and activation of pro-caspase-3 to caspase-3 in preclinical models9.
The hydrolysable CL2A linker conjugating the drug and the antibody enables intracellular and extracellular release of SN-38 upon internalisation by the target cancer cells. A bystander killing effect is also achieved to overcome antigen heterogeneity as SN-38 penetrates cell membrane and reaches the tumour microenviornment10.
Evidence of Efficacy and Safety
Promising efficacy and safety of SG in were demonstrated in a recent phase I/II multicentre trial, IMMU-132-01, involving 108 patients with heavily-pretreated refractory mTNBC. An overall response rate of 33.3% (including partial response in 33 patients and complete response in 3 patients, Figure 2) was achieved. The median duration of response was 7.7 months, whereas the median progression-free survival (PFS) was 5.5 months and the median overall survival (OS) was 13.0 months. Further, a clinical benefit rate of 45.4% was yielded by 10 mg/kg SG treatment11. Moreover, there was no cross-resistance to prior chemotherapy or immune checkpoint inhibitor was observed. The rate of treatment discontinuation due to adverse events (AEs) was 3% with no treatment-related deaths. The adverse events were readily manageable by granulocyte colony stimulating factor or dose modification. Based on the promising results of the IMMU-132-01 trial, the accelerated approval of SG for treating mTNBC was granted by the FDA in April 202012.
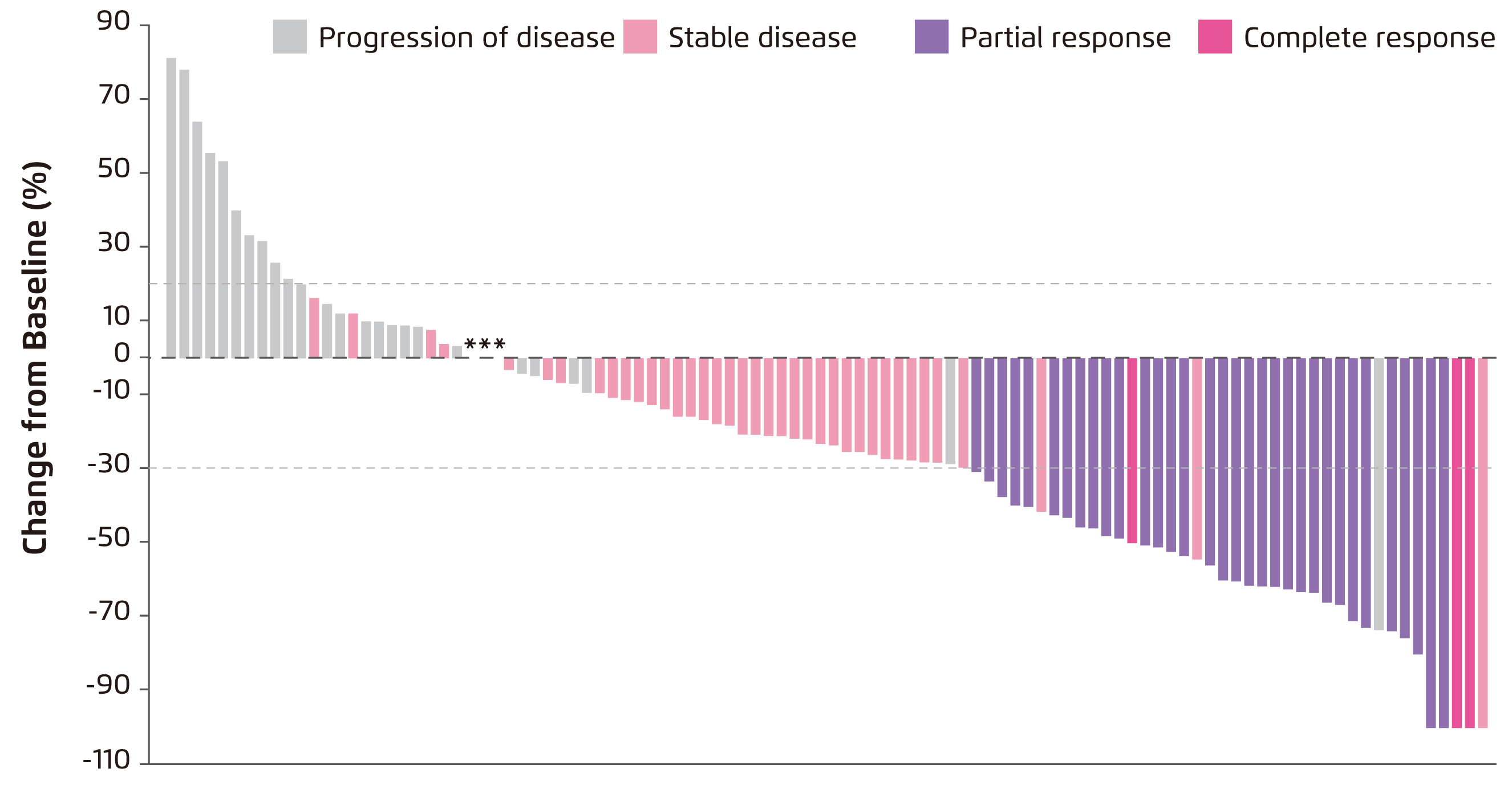
Figure 2. Waterfall plot showing change in tumour size in the IMMU-132-01 trial11
Future Prospects
Combined treatment of SG with existing therapies such as PARP inhibitors have demonstrated remarkable synergistic anti-tumour effect in TNBC cell lines and rodent models13. It has been reported that inhibition of PARP prevents the repair of camptothecin-induced stalled replication forks and hence sensitising cancer cells to TOPOI inhibitors14. Nevertheless, coupled interactions between PARP and TOPOI during the cell cycle need to be further clarified to validate the value of dual therapy consisting of PARP inhibitors and TOPOI inhibitors. Further research is warranted to investigate efficacy and safety of these attractive combination therapies in human. Remarkably, SG represents an exciting and ground-breaking advancement in novel treatments for mTNBC owing to its unique design and promising experimental findings. Further exploration on the pathophysiological role of Trop-2 in mTNBC and the correlation between Trop-2 expression and response to SG would facilitate better response prediction.
Acknowledgement
The author acknowledges the contribution of Ms Anna Yau of the School of Biomedical Sciences, CUHK in the preparation of this article.
References
1. Yeo. Hong Kong J. Radiol. 2015; 18: 111-8. 2. Anders et al. Oncology 2008; 22: 1233-9. 3. O’Reilly et al. BBA Clin. 2015; 3: 257-75. 4. Sahota et al. Expert Opin Biol Ther 2017; 17: 1027-31. 5. Kwa et al. Cancer. 2018; 124: 2086-103. 6. Tagawa et al. Ann Oncol 2019; 30: v851-934. 7. Ambrogi et al. PLoS One 2014; 9: e96993. 8. Li et al. Am. J. Cancer Res. 2017; 7: 2350-94. 9. Goldenberg et al. Oncotarget 2015; 6: 22496-512. 10. Zeybek et al. Sci Rep 2020; 10: 1-10. 11. Bardia et al. N Engl J Med 2019; 380: 741-51. 12. FDA. FDA grants accelerated approval to sacituzumab govitecan-hziy for metastatic triple negative breast cancer. 2020. 13. Cardillo et al. Clin Cancer Res 2017; 23: 3405-15. 14. Znojek et al. Br J Cancer 2014; 111: 1319-26.