
The Importance of Systemic Treatment Sequencing in Improving Survival for Patients with Advanced Liver Cancer
Dr.Catherine Frenette, Prof. Peter Galle, Dr.Richard Finn,
Prof. Jean-Frédéric Blanc, Dr. Kirhan Özgürdal, Prof. Ann-Lii Cheng, Dr. Tulio Pfiffer and Prof. Hui-Chuan Sun
Liver cancer is the 6th most common cancer diagnosis and the 4th most common cause of cancer deaths globally, whereas hepatocellular carcinoma (HCC) accounts for 75-85% of all liver cancers making HCC a major cause of cancer and cancer-related deaths1. Of note, liver cancer accounts for 43.6% of all liver-related deaths in Asia-Pacific region and the proportions in the USA and Europe are 33.9% and 39.4%, respectively2. Currently, multiple systemic therapies are available for HCC in both the first- and second-line setting. Of importance, timely move from locoregional treatment to systemic therapy and effective sequencing of systemic therapies would potentially provide survival benefit in patients with advanced HCC3. In a recent meeting, liver cancer clinicians from Europe and the United States addressed an International Expert Statement on recent changes in systemic treatment sequencing in advanced HCC aiming to improve overall survival for the patients.
Importance of Improving Survival in Patients with Unresectable HCC
Dr. Catherine Frenette
Medical Director of the Liver Transplantation Program
Scripps Green Hospital, USA
While liver cancer is one of the most common causes of cancer death, Dr. Frenette presented, based on the global cancer statistics in 2018, that the number of deaths caused by liver cancer surpassed cancers of breast, esophagus and pancreas, and was comparable to that of stomach cancer (Figure 1)4. Further, the incidence of liver cancer showed an increasing trend globally, with an increase by 38% from 2006 to 20165. Even worse is that liver cancer is generally diagnosed at a late stage, which significantly brings down the survival rate and the estimated 5-year mortality rate of the disease is up to 86%6. Practically, most diagnosed HCC are unresectable that less than 30% of HCC patients are eligible for potentially curative therapies7.
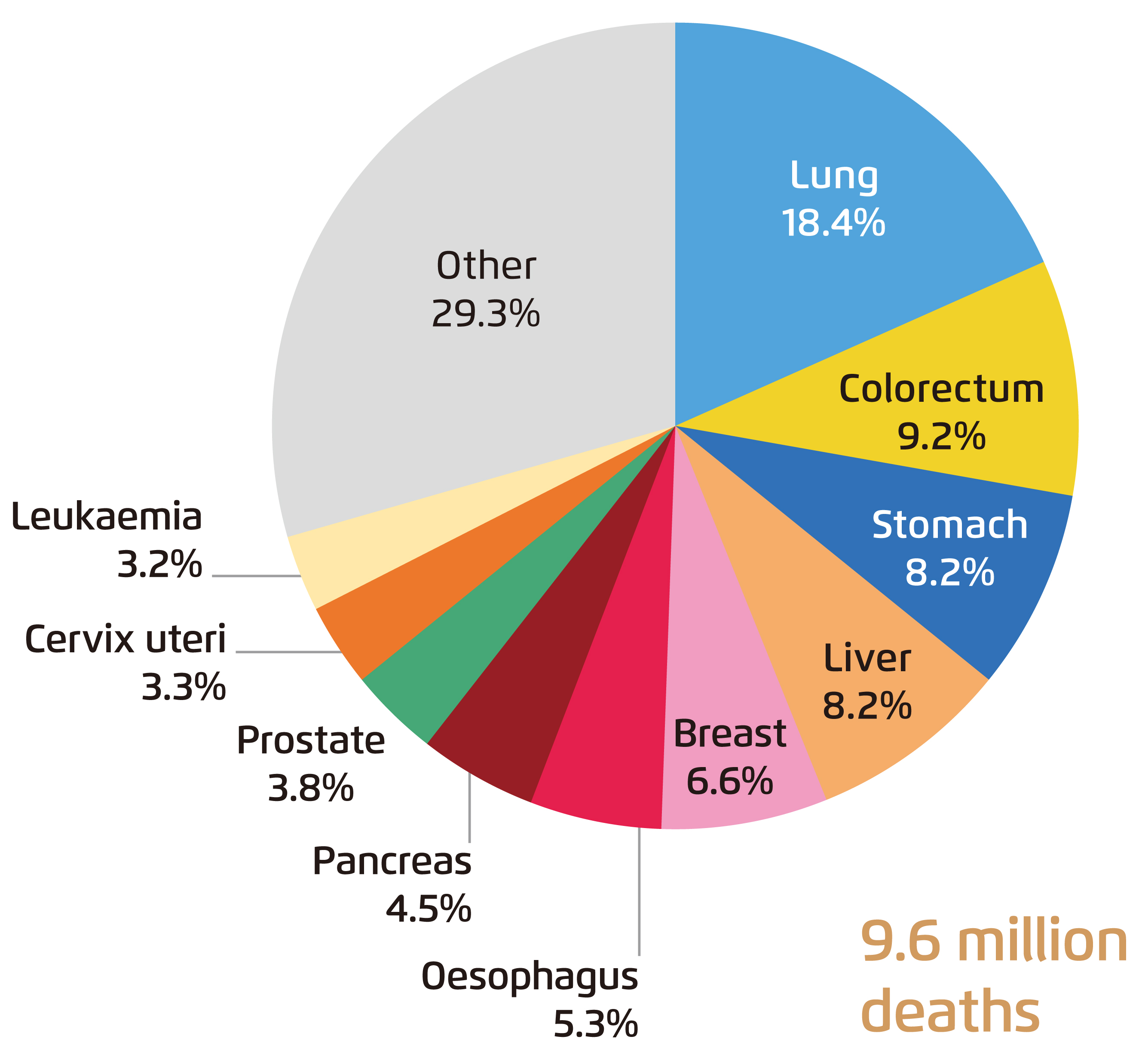
Figure 1. Global mortality by common cancers in 2018 4
In view of the recent increase in available systemic treatment options for unresectable HCC, Dr. Frenette addressed the need for optimising outcomes for HCC patients by re-evaluating of treatment practices for HCC including patient selection for each therapy and eligibility for each treatment option. In particular, she highlighted that timely initiation of systemic therapy and optimal sequencing of systemic treatments would potentially prolong overall survival for HCC patients by more than 2 years8.
On behalf of the expert panel, Dr. Frenette presented the International Expert Statement. The Expert Statement aims to highlight the values of systemic treatment sequencing for maximizing patient outcomes in HCC. There are 3 major focuses discussed in the Expert Statement, including 1) to highlight current challenge of managing patients with HCC, 2) to provide clear, straight-forward guidance on implementing a robust clinical approach to systemic treatment sequencing, and 3) to recommend an multidisciplinary team (MDT)-led approach to the effective treatment and care of patients with HCC to improve outcomes, including the role for patient education.
Changing Treatment Landscape and Importance of Systemic Therapy Sequencing
Prof. Peter Galle
Director, Department of Internal Medicine,
University Medical Center,
Mainz, Germany
While locoregional therapy, such as transcatheter arterial chemoembolisation (TACE), is currently the standard of care for Barcelona Clinic Liver Cancer (BCLC) intermediate-stage (BCLC-B) HCC and systemic therapy is essentially reserved for advanced disease (BCLC-C), Prof. Galle presented the importance of changing the current treatment landscape by early use of systemic therapy in the management of BCLC-B HCC.
Former trial demonstrated that TACE is effective only for a limited number of cycles and would deplete liver function in long run9. Prof. Galle addressed that patients with high tumour burden such as with multiple (>7) nodules or huge (>6 cm) nodules might not be good candidates for TACE9. These patients typically response poorly to TACE or easily become TACE-refractory. If continuing TACE till TACE refractoriness, liver function would plummet, devoiding chance for subsequent systemic therapy, emphasised Prof. Galle. He commented that systemic therapy is also useful in BCLC-B HCC. Essentially, administering systemic therapy before the decline in liver function may allow patients to receive multiple lines of treatment with the potential to extend overall survival. As demonstrated in the iconic Phase III SHARP and Asia-Pacific trials, first-line sorafenib treatment significantly improved median overall survival (OS) as compared to placebo in advanced HCC patients10,11.
Prof. Galle mentioned that change in HCC systemic treatment landscape is characterised by the emergence of successful clinical trials, including trials on single therapy or in combinations, leading to approval of various first-line and second-line therapies since 2017. He further outlined potential future treatment strategies for unresectable HCC including combinations of tyrosine kinase inhibitors (TKIs) with checkpoint inhibitors such as PD-L1. Moreover, combinations of checkpoint inhibition and addition of checkpoint inhibitors to combination therapies are potential strategies for managing unresectable HCC as well.
Prof. Galle expressed that there are more treatment options available for HCC nowadays compared to a decade ago. Currently, the first-line systemic therapy options for patients with HCC include sorafenib, lenvatinib and the combination therapy of atezolizumab and bevacizumab. Yet, clinical trial data on second-line treatment are available only upon progression and/or intolerance to sorafenib. To illustrate the survival benefit of effective sequencing of systemic therapies, Prof. Galle presented the findings of an analysis of the Phase III RESORCE trial, which showed that sorafenib treatment followed by second-line regorafenib significantly prolong median OS (26.0 months) as compared to sorafenib followed by placebo (19.2 months). Thanks to the 54% reduced risk of disease progression or death by regorafenib over placebo (progression-free survival (PFS) hazard ratio (HR): 0.46, 95% confidence interval [CI]: 0.37-0.56, one-sided p < 0.0001), the estimated 5-year survival rate yielded by sorafenib-regorafenib (16%) was higher than that yielded by sorafenib-placebo (3%)8 and such benefit in OS has been consistently, if not exceedingly, observed in a number of Asian real life cohorts12-14. Based on the substantial improvement in survival achieved by sorafenib-regorafenib sequence (Figure 2), Prof. Galle suggested offering the systemic therapy sequence to as many HCC patients as possible.
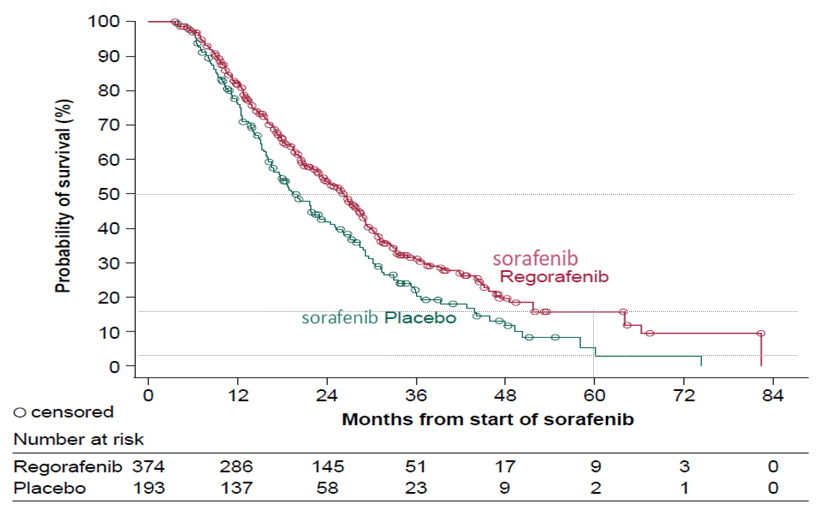
Figure 2. OS achieved by sorafenib-regorafenib in RESORCE trial8
Effective Treatment and Management Plan to Improve Survival in HCC
Dr. Richard Finn
Professor of Medicine
UCLA Medical Center, USA
Dr. Finn addressed that the goals of management of HCC include improving survival, optimising quality of life and minimising side effects proactively. Translating the goals into clinical practice, Dr. Finn outlined 3 focus areas: 1) planning, 2) proactivity, and 3) progression.
Planning refers to the selection of eligible patients for treatment and the development of a treatment plan including systemic treatment sequencing. Dr. Finn advised that accurate disease staging for patients in the first visit is crucial since outcomes of TACE depend not only on tumour burden, but also liver function and performance status. Essentially, the disease staging and treatment allocation should be discussed in MDTs, which include liver surgeons, radiologists, oncologists, and etc., to tailor individualised treatment options within the framework of accepted guidelines. Former report suggested that, as compared to traditional approach, HCC patients managed with MDT approach had earlier and more accurate diagnosis and received better treatment, leading to significant improved OS (Figure 3)15.
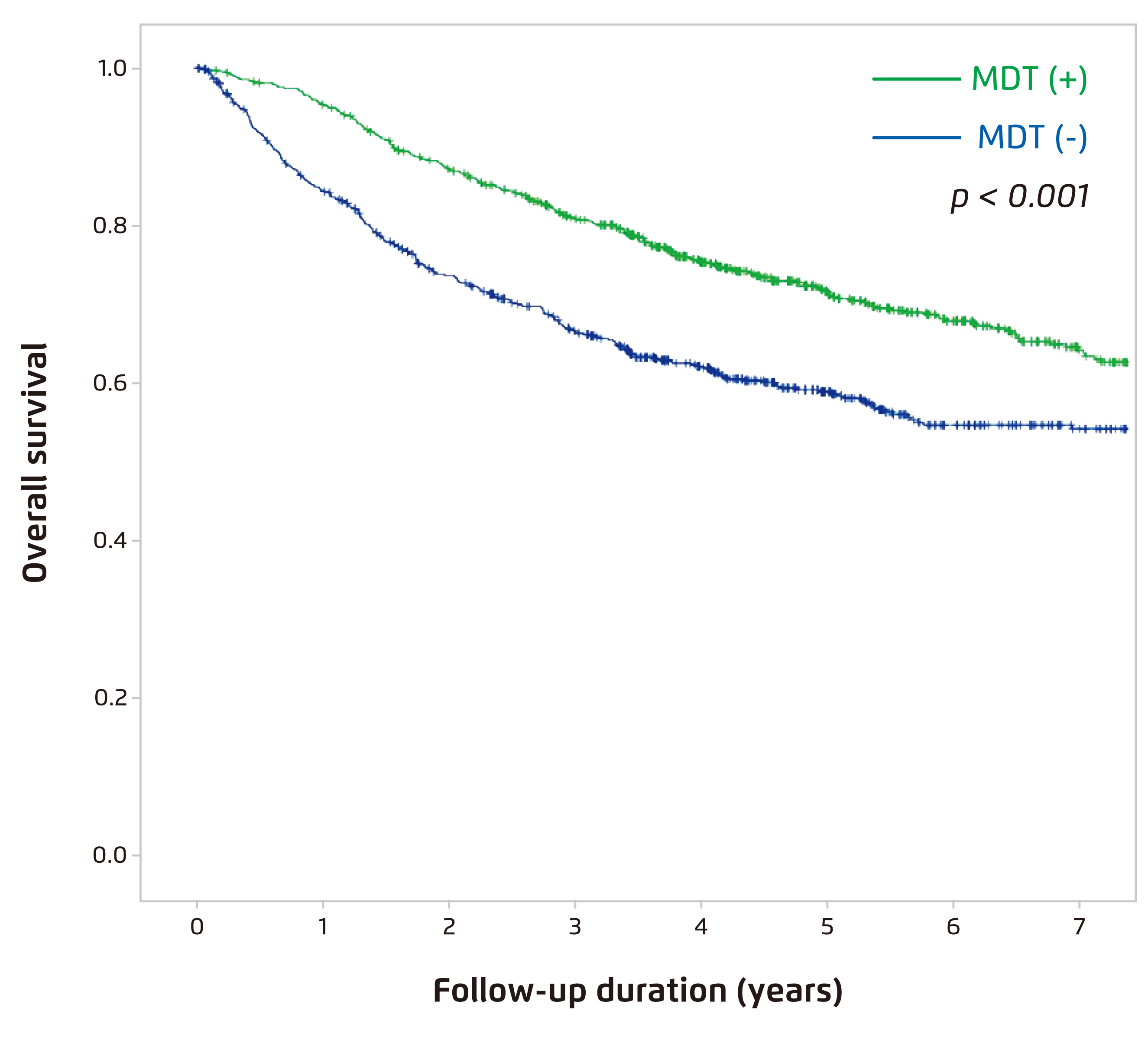
Figure 3. OS in newly diagnosed HCC patient managed with (n=738) versus without (n=5,881) MDT from 2005 to 201315
Proactivity means proactively managing underlying liver disease, assessing TACE efficacy and monitoring liver function and toxicity. For the intermediate-stage HCC patients on TACE, assessing refractoriness and liver function is crucial. Of note, TACE efficacy is defined by complete or partial response, but stable disease is not sufficient16. Dr. Finn emphasised that maintaining adequate liver function would ensure patient eligibility for potential subsequent systemic therapy. As a number of systemic treatments can be used in sequence, a timely switch from TACE to systemic therapies would allow multiple lines of therapy and hence improve OS for patients who are no longer eligible for TACE or progress on TACE. Besides, proactively management of adverse events (AEs) enables early control of tr eatment related-AEs and tailored dosing, improving tolerability and prolonging treatment duration. Appropriate pre-treatment and patient education would encourage early detection of AE and timely management.
Regular assessing disease progression through clinical observations, laboratory and imaging data, is essential. Based on the assessment findings, patients can be transferred to the next phase of treatment before deterioration of physical condition. Dr. Finn commented that systemic therapies for HCC are effective in delaying disease progression. Besides, Dr. Finn advised that keeping close communication with patients is essential. For instance, knowledge on benefits of treatments as well as risks of potential AEs and expectations on outcome should be provided to patients.
Recommended Approach for the Management of HCC by the Panel
While majority of patients are diagnosed with unresectable HCC, TACE is the current standard of care for intermediate-stage patients. Nonetheless, the tumour response to TACE is highly heterogeneous and a shift in the treatment paradigm is suggested. The panel recommended proactively providing systemic treatment for intermediate-stage HCC patients who do not respond to TACE or would potentially benefit from systemic therapy instead of waiting for disease progression. Essentially, the panel stressed that locoregional therapy would damage liver function, particularly in patients with underlying liver cirrhosis, whereas inappropriately continuing locoregional therapy would result in ineligible for systemic therapy. Thus, transition from locoregional therapy to systemic therapy at the time of TACE refractoriness was recommended by the panel.
Switching gear to systemic treatment, despite recent approval in first line settings data guiding second-line treatment after progression or intolerance on lenvatinib or atezolizumab/bevacizumab are inadequate. Thus, the panel reminded to consider the appropriate therapy after the first-line treatment options. Essentially, effective sequencing of available systemic therapies would substantially increase survival for patients with HCC. To conclude, the panel advocated that the management of HCC is complex and the involvement of a multidisciplinary team is critical during the course of treatment.
References
1. Aly et al. Hepatic Oncol 2020; 7: HEP27. 2. Sarin et al. Lancet Gastroenterol. Hepatol. 2020; 5: 167-228. 3. Bruix et al. Lancet 2017; 389: 56-66. 4. Bray et al. CA Cancer J Clin 2018; 68: 394-424. 5. Fitzmaurice et al. JAMA Oncol 2018; 4: 1553-68. 6. Sachdeva et al. World J. Hepatol. 2015; 7: 2080-90. 7. Llovet. Nat Rev Clin Oncol 2015; 12: 408-24. 8. Finn et al. J Hepatol 2018; 69: 353-8. 9. Kudo et al. Liver Cancer 2018; 7: 215-24. 10. Llovet et al. N Engl J Med 2008; 359: 378-90. 11. Cheng et al. Lancet Oncol 2009; 10: 25-34. 12. Yoo et al. Invest New Drugs 2019; 37: 567-72. 13. Yoo et al. Liver Int 2020; 40: 2263-71. 14. Ogasawara et al. Invest New Drugs 2020; 38: 172-80. 15. Sinn et al. PLoS One 2019; 14: e0210730. 16. Galle et al. J Hepatol 2018; 69: 182-236.





