
Director and Professor of S.H. Urology Centre,
Department of Surgery,
The Chinese University of Hong Kong (CUHK)
Prostate cancer (PCa) is a complex disease that affects millions of males, globally. It is the 3rd most common cancer in males and the 4th leading cause of male cancer-related deaths in Hong Kong1. The aetiology of PCa is believed to be related to several risk factors which includes the male gender, old age (aged ≥50 years), positive family, obesity, diet and ethnicity2. More important, PCa often follows an indolent clinical course since patients with PCa often remain asymptomatic during the early stages of the disease3, thus, at diagnosis, the disease is often at late stages, requiring extensive treatment4. Therefore, to understand the complex nature of PCa, we have invited Professor Ng Chi Fai, a specialist, and director of SH Ho Urology Centre at the Chinese University of Hong Kong (CUHK) to discuss the reasons behind the late diagnosis of the disease, in addition to provide an overview on the treatment in early and advanced stages of the disease, particularly the use of robot-assisted radical prostatectomy (RARP), and the integration of artificial intelligence (AI) into clinical practice.
Unveiling the Hidden Danger of Prostate Cancer
Prostate is an accessory reproductive organ in males, primarily responsible to complement the essential secretions to semen, and to keep the sperm viable. Notably, the adult prostate is divided into central, transitional and peripheral regions, with 95% of PCa cases occurring at the peripheral zone of the prostate5 with an acinar origin6. More importantly, PCa is common in male, accounting for 1 in every 14 cancers diagnosed globally, and 15% of all cancers reported in males7. Locally, PCa is the 3rd commonest cancer in males and the 4th leading cause of male cancer-related deaths1. Naturally, PCa is associated with a number of risk factors as Prof. Ng explained that these risk factors include the male gender, old age (males ≥ the age of 50), positive family history, obesity, diet (westernised diet), and ethnicity (higher prevalence in Blacks and Caucasians)2. Here, Prof. Ng added that male with a first-degree relative (father or brother) with PCa have twice the risk of developing PCa compared to the general population2.
Fortunately, most localised PCa run a relative indolent course, with population studies indicating it as slow progression disease with limited aggressiveness3. Nevertheless, patients with early PCa often remain asymptomatic, and up to 14% of patients with PCa demonstrate metastatic disease at the time of diagnosis4. Why? Prof. Ng highlighted that even though early diagnosis of PCa remains the cornerstone, most patients with early PCa may not recognise symptoms until they are well into the late stages of the disease, requiring aggressive treatment. In fact, up to 75% of patients with PCa experiences metastatic bone disease which often leads to an increased risk for skeletal-related events (SREs), including pathological bone fractures, spinal cord compression, and hypercalcaemia8. More worryingly, recent population-based studies have reported that 86% of the patients associated PCa with symptoms, but only 1% were aware that it could be asymptomatic5. Prof. Ng stressed a salient point that males with early staged disease often hesitate to seek help early compared to female, and they only seek help when the disease is in advanced stages9.
An Era of Precision Oncology
The standard diagnostic tools for detecting PCa include a digital rectal examination (DRE), followed by a blood-based analysis of prostate specific antigen (PSA) and imaging. What is DRE and how does it help with the diagnosis? DRE is a physical palpation of the prostate to assess the gland enlargement, texture and stiffness with a positive predictive value in detecting PCa of 5-30% in males with PSA levels of ≤2 ng/ml10. A prostate biopsy (needle biopsy) is indicated for an abnormal DRE result, which is associated with a worse differentiation grade, but considered as a definitive diagnosis. What about the serum PSA? Serum PSA may complement prostate cancer detection efforts, but like DRE, PSA testing can be abnormal without PCa being present (false-positive) and can be normal, though rare, despite the presence of PCa (false-negative)10. Prof. Ng reminded us that PSA is a very sensitive but relatively non-specific and imprecise screening tool, as both benign and malignant processes will cause an elevation in serum PSA levels4. Hence, multiple factors should be considered in patients with a rise in serum PSA. Factors that influence the survival of PCa patients are dependent on the clinical stage of the tumour, the histological grade of tumour, patient’s comorbid-adjusted life expectancy, and the PSA level11.
After the initial diagnosis, the disease is usually staged (stage I-IV) and treatment depends on the tumour's stage and grade. For instance, radical prostatectomy or external beam radiation therapy is considered for patients with intermediate to high-risk localized PCa. Contrary to this, brachytherapy or focal therapy, such as cryotherapy, are treatment options for patients with more focal disease and low to intermediate-risk diseases 12, according to Prof. Ng. He elaborated further that patients, due to the slow-growing nature of PCa, patients, particularly the elderly, with low-risk to very low-risk diseases may consider active surveillance13. What about the treatment options for advanced PCa? It has long been known that PCa is unique since it is highly dependent on androgen for growth and progression; therefore, androgen deprivation is an effective therapeutic strategy widely used in clinical practice14. Prof. Ng explained further that androgen deprivation therapy (ADT) often induced PCa regression and prolonged survival for these patients15. However, he warned like other hormonereplacement therapies (HRTs), patients may experience adverse events associated with the ADT, which include increased cardiometabolic risk, hot flushes, loss of libido, erectile dysfunction, depression, anaemia, and osteoporosis16; fortunately, these are manageable adverse events.
The Golden Age of Robotic Surgery in Prostate Cancer Management
Radical prostatectomy is the recommended surgical treatment for clinical localised PCa, which provides long-term oncological control. Traditionally, open retropubic radical prostatectomy (RRP) had often been utilised, but due to the complexity of the pelvic anatomy, particularly, because the prostate lies deep and is often hard-to-reach17. Thus, a minimally invasive method such as laparoscopic prostatectomy was initially developed but failed to take off as a mainstream treatment due to the technical difficulties associated with the technique17. The shortfalls related to the laparoscopic prostatectomy were overcome by robot-assisted surgery that has since transformed the landscape of urological interventions. One such advance includes the use of RARP for the management of PCa. The procedure was introduced in 2002 by Binder and marked a significant milestone by combining the advantage of minimally invasive radical prostatectomy with enhanced surgeon ergonomics and improved technical ease in vesicourethral anastomosis reconstruction18. Prof. Ng explained that RARP has generally been accepted to have a lower estimated blood loss and shorter hospital stays with lower intraoperative adverse event rates when compared to traditional radical open prostatectomy19.
These findings have been substantiated in a prospective multicentre controlled trial, study by Lantz et al., 2021 that evaluated the functional and oncological long-term outcomes 8 years after robot-assisted laparoscopic prostatectomy (RALP) and open retropubic radical prostatectomy (RRP). The primary endpoint of the study was urinary incontinence, and the results showed that the incidence of urinary incontinence was not significantly different at 8 years after surgery between RALP and RRP (27% vs 29%). However, the incidence of erectile dysfunction was significantly lower in the RALP group (66% vs 70%; adjusted risk ratio [aRR] 0.93, 95% confidence interval [CI] 0.87-0.99)20. Furthermore, prostate cancer-specific mortality (PCSM) was significantly lower in the RALP group at 8 years after surgery (Figure 1).
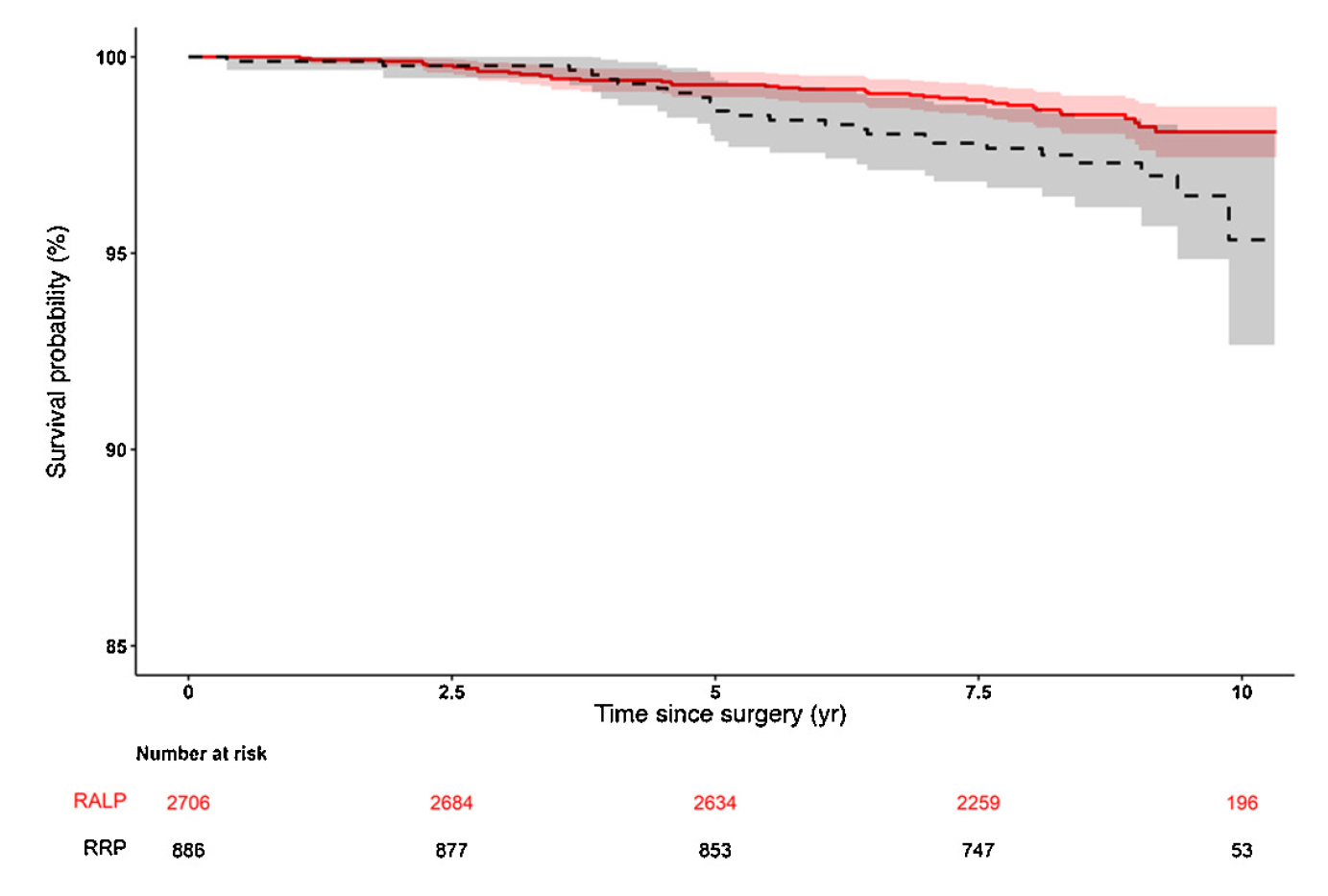
Figure 1. Prostate cancer-specific mortality for RALP versus RRP and for D’Amico20. Stratification by D’Amico risk groups (low, intermediate, and high risk). RALP = robot-assisted laparoscopic prostatectomy; RRP = open retropubic radical prostatectomy.
The conclusion reached here was that robot-assisted minimally invasive techniques are safe in the long-term20. Similarly, a systematic review and meta-analysis by Wang et al., 2023 on prospective studies comparing RARP with open radical prostatectomy concluded that RARP was superior to open radical prostatectomy in terms of hospital stay, blood loss, transfusion rate, complication, nerve sparing, postoperative erectile function recovery and biochemical recurrence21.
Artificial Intelligence, a New Tool Against Prostate Cancer
AI application has enabled remarkable advancements in healthcare delivery and to improve accuracy, as well as the efficiency of diagnostic imaging interpretation22. Currently, there is a widespread potential of AI in the field of Urology, particularly in the diagnosis, and the treatment of PCa, Prof. Ng commented. He elaborated that many studies have shown that AI-powered systems can accurately detect PCa and help predict patient outcomes, leading to a higher potential to improve patient care23, locally. To showcase the potential AI in clinical practice, Saha et al., 2024 performed an international, paired, non-inferiority, confirmatory study that compared the performance of AI systems at detecting clinically significant PCa on MRI in comparison with radiologists using the Prostate Imaging-Reporting and Data System version 2.1 (PI-RADS 2.1) and the standard of care in multidisciplinary routine practice. Interestingly, among 10,207 examinations conducted from January 1st, 2012, through December 31st, 2021, 2,440 cases had histologically confirmed Gleason grade 2 or greater PCa. The results demonstrated that AI system was superior to radiologists using PI-RADS (2.1), on average, at detecting clinically significant PCa and comparable to the standard of care24. These findings demonstrated the potential of AI as a supportive tool within a primary diagnostic setting.
The role of AI in diagnosing PCa has recently been explored in a retrospective study by Hamm et al., 2023. The aim of this study was to develop an explainable AI (XAI) model for clinically significant PCa diagnosis at biparametric magnetic resonance imagining (MRI) using Prostate Imaging Reporting and Data System (PI-RADS) features for classification justification. Among 1,244 males (median age of 67 years) with 3,260 prostatic lesions (372 lesions with Gleason score of 6; 743 lesions with Gleason score of ≥ 7; 2,145 benign lesion), XAI reliably detected clinically significant PCa in internal and external test sets with a sensitivity of 93% and an average of one false-positive findings per patient. Furthermore, the XAIassisted readings improved the confidence of non-experts in assessing PI-RADS 3 lesions, reducing the reading time by 58 seconds (p=0.009). These results demonstrated that AI integration in clinical practice can help reliably detect, and classify clinically significant PCa and improve the confidence and reading time of non-expert in the field of urology25. Here, Prof. Ng added that AI integration has already been implemented at the CUHK as a trial, and hopefully, this will invariably support clinicians in many different specialities. He also reminded us that PCa is becoming more common locally with an ageing population. Thus, it is crucial for clinicians to use different diagnostic modalities to identify the disease and treat it as early as possible to reduce the disease burden.
References
1. GHealth Do. Prostate Cancer. 2024. https://www.chp.gov.hk/en/healthtopics/content/25/5781.html (accessed 29/08/2024 2024). 2. Leslie SW, et al. StatPearls Publishing LLC.; 2024. 3. Rosario E, et al. StatPearls Publishing LLC.; 2024. 4. David MK, et al. StatPearls Publishing LLC.; 2024. 5. Gnanapragasam VJ, et al. BMC Medicine 2022; 20(1): 264. 6. Wasim S, et al. Int J Mol Sci 2022; 23(22). 7. James ND, et al. The Lancet 2024; 403(10437): 1683-722. 8. Broder MS, et al. Support Care Cancer 2015; 23(1): 237-47. 9. King-Okoye M, et al. Psychooncology 2019; 28(6): 1321-7. 10. Rebello RJ, et al. Nature Reviews Disease Primers 2021; 7(1): 9. 11. Board PDQATE. National Cancer Institute (US); 2002. 12. Mercader C, et al. Aging Male 2020; 23(5): 1460-6. 13. Brawley S, et al. Am Fam Physician 2018; 97(12): 798-805. 14. Teo MY, et al. Annu Rev Med 2019; 70: 479-99. 15. Shore ND, et al. Prostate 2020; 80(6): 527-44. 16. Perlmutter MA, et al. Rev Urol 2007; 9 Suppl 1(Suppl 1): S3-8. 17. Simsir A, et al. Pak J Med Sci 2021; 37(1): 167-74. 18. Brime Menendez R, et al. World Journal of Urology 2024; 42(1): 336. 19. Chandrasekar T, et al. Transl Androl Urol 2018; 7(Suppl 1): S120-s3. 20. Lantz A, et al. Eur Urol 2021; 80(5): 650-60. 21. Wang J, et al. J Robot Surg 2023; 17(6): 2617-31. 22. Baydoun A, et al. Prostate Cancer Prostatic Dis 2024; 27(1): 37-45. 23. Chu TN, et al. Curr Urol Rep 2023; 24(5): 231-40. 24. Saha A, et al. Lancet Oncol 2024; 25(7): 879-87. 25. Hamm CA, et al. Radiology 2023; 307(4): e222276.





