

President, International Society for Peritoneal Dialysis
Consultant Nephrologist, Imperial College Renal and Transplant Centre Hammersmith Hospital, UK
Along with the rising global prevalence of end-stage renal disease (ESRD), the number of patients receiving maintenance dialysis has increased dramatically in recent decades1. Practically, dialysis outcomes and patient experiences are determined by a wide range of factors, such as the availability of resources, the timing of dialysis initiation, and dialysis modality. While the clinical benefits of peritoneal dialysis (PD) have been well-established, the impact of increasing dialysis exchanges and the related time burden on patients' quality of life (QOL) has also been recognised. This realisation leads to the advocacy of high-quality goal-directed dialysis2. This symposium celebrated the 25th Anniversary of the Automated Peritoneal Dialysis (APD) Program in Hong Kong on 11th March 2023 and Prof. Edwina Brown highlighted the role of APD in prescribing personalised PD, in addition, shared her insight on the future development of APD.
In healthcare settings, the delivery of PD primarily focuses on small solute removal and ultrafiltration as highlighted by the International Society for Peritoneal Dialysis (ISPD) guidelines in 2006. However, the recommendations had, in fact, stated that the adequacy of dialysis should be interpreted clinically rather than by targeting only solute and fluid removal3. With the increasing awareness on the importance of patient's QOL, the Kidney Disease Improving Global Outcomes (KDIGO) advocated the change in terminology from “adequate dialysis” to “goal-directed dialysis” in 2018. This explicitly refers to shared decision-making between the patient and caring team aimed to establish realistic care plan that will allow the patient to meet their own life goals; in addition, to allow the clinician to provide personalised, high-quality dialytic care1.
Prof. Brown highlighted that dialysis affects the targeted outcomes addressed in goal-directed PD, this may include the symptom burden, nutritional status, activity level, work capacity, and social engagement1, the impacts are mostly indirect. Based on the clinical emphasis above, the ISPD compiled new practice recommendation in 2020, referred to as “high-quality goal-directed PD”. Prof. Brown presented the new recommendation aimed to provide the best health outcome for individuals on PD in terms of maintaining their clinical well-being, QOL, ability to meet life goals, as well as minimising treatment burden2. “(From the updated ISPD recommendation), PD should be prescribed using a shared decision making approach between the patient on PD, the caregiver, and the caring team,” according to Prof. Brown. The key ISPD recommendations for prescribing high-quality goal-directed PD are illustrated in Figure 1.
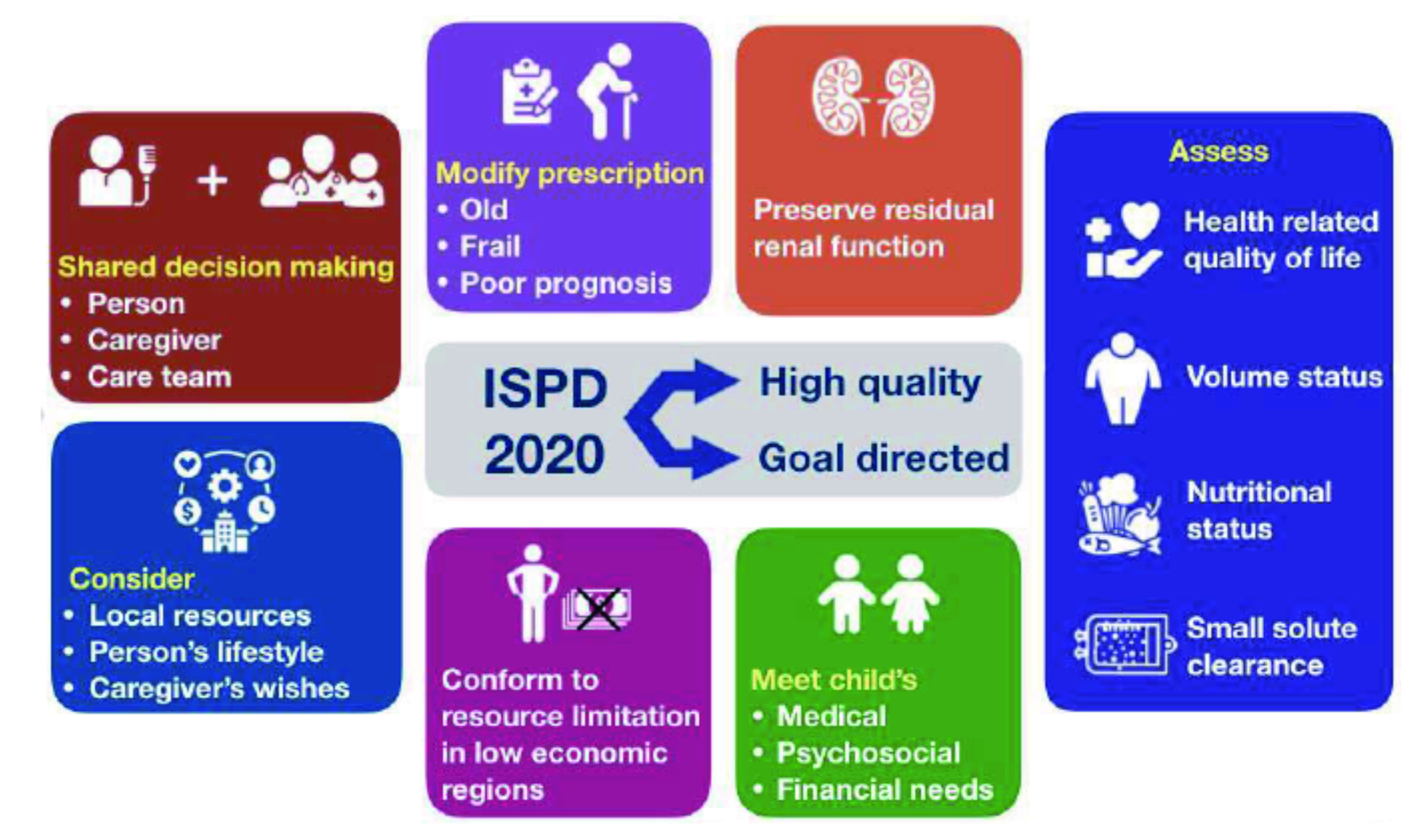
Figure 1. Key ISPD recommendations for prescribing high-quality goal-directed PD (figure presented by Prof. Brown)
Patient burnout has been reported as a major cause of technique failure in PD. Thus, personalised PD may potentially prolong the time on PD by reducing dialysis burden4. By utilising a cycler machine, APD allows the exchange of larger amounts of fluid over shorter periods, hence dialysis may potentially be performed intermittently, thereby allow patient a more personalised time. In addition to a reduced dialysis burden, patients on PD may also be less prone to the risk of peritonitis5. In this regard, APD would improve PD outcomes by implementing high-quality goal-directed PD.
APD therapy was first introduced during the 1960s. It was then improvised by equipping it with the reverse osmosis system, which eliminated the need for large bottles used for containment of PD fluid, thereby, leading to a significant reduction in the physical size of the device. Nowadays, glass bottles are replaced by plastic bags and the development of the Tenckhoff catheter permitted a permanent access to the peritoneal cavity. Along with continuous ambulatory PD (CAPD), APD contributes to safer and simpler PD therapy for ESRD patients5.
Despite the benefit of having more dialysis-free period, when prescribing PD modality, it is crucial to consider the pros and cons with regard to each patient's co-morbidities, physical disabilities, social activities, psychological readiness, and treatment cost-benefit. Prof. Brown suggested APD being preferable for patients who are high transporters with poor ultrafiltration on CAPD, whereas frequent PD exchanges with APD helps to improve the total ultrafiltration process in these patients5. Moreover, patients on CAPD may require 5 or more bag changes, especially those with a large body mass with ESRD and those with no residual renal function. Therefore, APD should be considered in such cases5.
However, APD has its own limitations. According to Prof. Brown, APD is not suitable for patients with poorly functioning catheters. On the other hand, patients are often required to spend a long time on the APD machine, sometimes >8 hours, thereby limiting the APD practicality. “It's a journey of 7 nights per week, similar to being on shift pattern at work,” Prof. Brown described. Moreover, some patients may complain of sleep disturbances since the machine can be noisy. Furthermore, patients also require to reserve more storage spaces at home for PD fluid. APD may make travelling difficult for patients, therefore, they may need to change to CAPD. “Choosing the appropriate PD modality is not always straightforward,” Prof. Brown commented.
Anuric patients are a difficult group to dialyse effectively using CAPD. To visualise the clinical benefits of APD, Prof. Brown presented the findings of the European APD Outcome Study (EAPOS, 2003), which involved 177 anuric patients on APD from 26 European centres in 13 countries. The APD prescription was adjusted at the physician’s discretion to aim for creatinine clearance (Ccrea) ≥60 L/week per 1.73m2 and ultrafiltration ≥750 ml/24hr during the first 6 months6.
The results showed that 78% and 74% of patients achieved Ccrea and ultrafiltration targets, respectively in 1 year. The median drained dialysate volume was 16.2 L/24hr, with 50% of patients using icodextrin. The patient survival was 78% and technique survival was 62% at year 2 (Figure 2). Moreover, the results suggested that older adults (>65 years), worse nutritional status (Subjective Global Assessment grade C), diabetic status, and lower ultrafiltration (<750 ml/24hr) at baseline were all associated with significantly worse patient survival6. While anuric patients with high peritoneal solute transport have a poor prognosis on CAPD, the targets in sufficient small solute clearance and ultrafiltration can be successfully achieved by using APD. The findings in EAPOS demonstrated the importance of ultrafiltration in contrast to small solute clearance as an outcome parameter for PD and, essentially, indicated that the survival of anuric patients on APD was similar to other patients on dialysis.
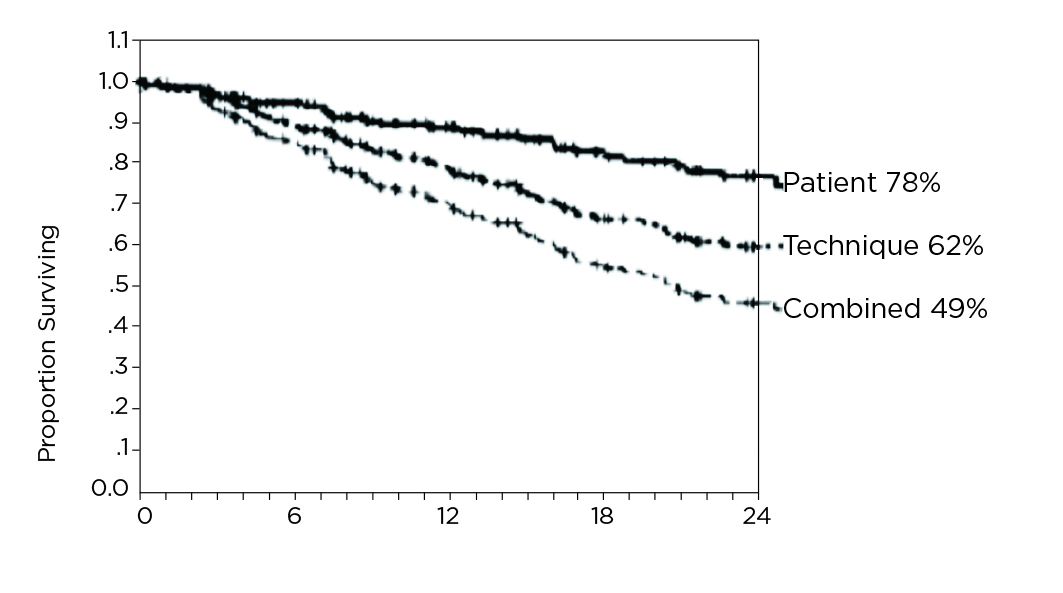
Figure 2. Kaplan Meier plots of patient survival at 2 years6
Blake et al. (2020) proposed that incremental PD is a strategy by which less than standard “full-dose” PD is prescribed to patients initiating the PD, so that the combination of residual renal and peritoneal clearance achieved is sufficient to achieve individualised clearance goals7. The strategy is initiated by understanding that the residual kidney function contributes to small solute removal. The PD prescription is increased over time as the residual kidney function declines, thus incremental PD is not considered as a palliative measure or a strategy under financial constraints. Furthermore, since less dialysate is used in incremental PD, the strategy contributes to a lower operating and overall cost for the patient. In addition, the strategy puts less workload on both the patients and their caregivers who assist them and is associated with less peritoneal glucose exposure7.
Prof. Brown highlighted that the incremental PD strategy is dependent on shared decision making with the patients. She outlined that the reduced prescription is dependent on the residual kidney function, whereas application of either CAPD or APD is based on the specific lifestyles, and conditions of the patient. During follow-up, the patient’s well-being, blood test results, urine output, and clearance are assessed to ensure that the sum of peritoneal and renal clearance meet the clearance target. In cases where incremental increase in PD prescription is required or a change in modality is considered beneficial, the decision will often be discussed with the patients and their families prior to commencement of the adjustments. Otherwise, the original prescription remains.
Prof. Brown illustrated the importance of shared decision making using a clinical case of a 56-years old male diabetic patient. The serum creatinine level of the patient was 650 µmol/L, with a glomerular filtration rate (GFR) of 7 and blood pressure of 154/92. The patient became symptomatic and progressively developed oedema. However, the patient ignored the symptoms and continued his occupation as a bus driver.
He was reluctant to start PD as he had difficulties in storing a large amount of PD fluid in his small apartment. Moreover, he expressed concern that he may not be able to continue his work after starting PD treatment. Therefore, Prof. Brown and her colleagues evaluated the patient's serum creatinine levels and his well-being during visits. After evaluation, they explained the potential outcomes of delayed PD to the patient and the risk of patient having to face an increased number of treatment days and exchanges had he not commenced on PD. The patient eventually agreed to start the PD. Taking the patient's concerns into consideration, Prof. Brown initiated the patient on a very low dose of 2 exchanges for 5 days regimen so that the patient was not required to stockpile a large amount of PD fluid and to minimise the impact of PD on his daily life. According to Prof. Brown, the patient showed significant improvement after commencing PD in approximately 6 weeks.
Prof. Brown suggested that assessing QOL, symptoms and depression status are helpful when evaluating the patient’s well-being. In addition, she highlighted the importance of assessing the volume control and solute removal. Moreover, non-dialysis factors such as co-morbidities should be considered2. “We need to know much more about the patients on PD and what really matters to them,” Prof. Brown emphasised. Of importance, when there is a change in PD prescription, it is crucial to provide patient different ways in which prescription can be changed, and their associated impact on patient's daily life.
Prof. Brown highlighted the potential role of remote patient monitoring, particularly during pandemics. Handling large amount of APD data for every single patient in a clinical unit can be difficult for nurses, therefore, utilising cycler machines can help store APD medical records, monitor the time course, and allow treatment review. Essentially, the output measurements of body weight and blood pressure are also feasible5. Prof. Brown explained that remote patient monitoring provides greater support for patients on PD and facilitates early identification of acute problems. It also helps reduce visits to PD centres and hospitalisation for patients. Very recently, the innovative use of an automated wearable artificial kidney, which utilises sorbent technology and requires only 1-2L PD fluid per day, has attracted the interest of clinicians. Nonetheless, more clinical trial data is required to confirm the performance of these devices. Remarkably, Prof. Brown suggested that streamlining regulatory approval in different regions and scaling up the production of PD equipment would help facilitate the application of new PD technology.
Lifestyle reasons may make APD the preferred modality for patients on PD. Accordingly, Prof. Brown presented the approach of implementing APD in personalised prescription to achieve the treatment goal. In the case of a 35-year-old male patient who started CAPD 12 months ago, with 3 exchanges for 6 days. The patient's condition improved initially, and he continued to work at the coffee bar. Due to unforeseen circumstance, the patient returned to clinic at a later date with exhaustion and limited exercise tolerability due to persistent shortness of breath, and reduced urine output. His blood pressure was 175/113, and oedema was prevalent. The serum creatinine was 1,150 µmol/L which promoted Prof. Brown to increase his prescription to 4 exchanges for 6 days and changed to APD for about 6 months when he received a successful kidney transplant.
In another clinical case of a 78-year-old patient on CAPD for 5 years, with 3 exchanges for 6 days. The patient remained independent and went for walks daily. He was also able to attend concerts. Inadvertently, his treatment was affected by the COVID pandemic leading to decreased urine output, declined Kt/V from 2.5 to 1.4, and serum creatinine of 750 µmol/L. Prof. Brown recommended assisted APD for this patient. However, patient refused. Considering the patient's age, and limited symptoms and being compos mentis, Prof. Brown decided not to proceed with prescription change.
While summarising the 2 cases, Prof. Brown emphasised that APD can prolong the patient’s time on PD while waiting for renal transplant and reduce the dialysis burden for many patients, but not everyone. In conclusion, Prof. Brown emphasised that the decision on a PD modality should be part of the shared decision making, which should include the PD team, patient on PD, and their family.
References
1. Chan et al. Kidney Int 2019; 96: 37–47. 2. Brown et al. Perit Dial Int 2020; 40: 244–53. 3. Lo et al. Perit Dial Int 2006; 26: 520–2. 4. Navaratnarajah et al. Perit Dial Int 2021; 41: 49–56. 5. Mizuno et al. Ren Replace Ther 2016; 2: 1–13. 6. Brown et al. J Am Soc Nephrol 2003; 14: 2948–57. 7. Blake et al. Perit Dial Int 2020; 40: 320–6. 8. Brown et al. Nephrol Dial Transplant 2022; 37: 2080–9. 9. Iyasere et al. Clin J Am Soc Nephrol 2016; 11: 423–30.





