Glaucoma is the second leading cause of blindness globally characterised by changes in the optic disc and visual field defects 1. Elevated intraocular pressure (IOP) is an important factor that leads to the death of retinal ganglion cells and their axons in glaucoma. In addition, the disease is often asymptomatic, and painless vision loss is the end-product of glaucoma. Therefore, early monitoring is required to prevent irreversible changes and halt the disease progression. Currently, there are no portable devices available to monitor continuous IOP change, particularly during sleep. Therefore, recent research has highlighted the viable use of smart contact lenses in detecting, monitoring, and treating glaucoma.
Glaucoma is a heterogeneous group of diseases that causes progressive degeneration of the optic nerve (cranial nerve II) and retinal nerve fibre layers, resulting in permanent loss of peripheral or central vision 2. It is considered one of the most common causes of irreversible blindness that manifest in those aged ≥ 40. There are around 64.3 million people affected by glaucoma worldwide, with 8.4 million blinded by the disease 1,3. Are there any risk factors for glaucoma? Yes, they include advanced age, elevated intraocular pressure, high myopia (short-sighted), family history of glaucoma and ethnicity 4.
Ethnicity is an interesting factor as epidemiological studies have suggested Black, Asians, and Hispanics have greater baseline severity compared to Whites counterparts for developing glaucoma, particularly the primary open-angle glaucoma (POAG) type 5,6. Glaucoma is classified broadly as congenital and acquired types. The congenital types include true congenital, infantile, and juvenile, whereas the acquired types include primary and secondary glaucoma. The primary and secondary types are often subclassified into open angle and angle closure types 7. The most common type is the primary open-angle glaucoma (POAG) in adults, which often remains asymptomatic until a substantial amount of optic nerve is damaged before signs and symptoms are prevalent 7.
But what is the cause of glaucoma then? Currently, the exact aetiology of glaucoma remains unknown but is believed to be mediated by the elevated pressure in our eyes, also known as “elevated intraocular pressure.” Glaucoma typically manifests over a period causing painless damage to the optic nerve through the ineffective drainage system in the eye. There are several postulated theories on the cause of glaucoma, with some suggesting the decrease in outflow or increased resistance to outflow of aqueous humour, the clear liquid within our eye. This gradually causes IOP to rise, thereby damaging the optic nerve ganglion cells in retinal nerve fibre layer. Furthermore, the raised IOP also impedes blood flow to the optic nerve fibres, leading to ischaemia of the nerve tissues, as illustrated in Figure 1 2. Currently, there is no treatment available to reverse the vision loss due to glaucoma, and the primary treatment aim is to prevent further deterioration 7.
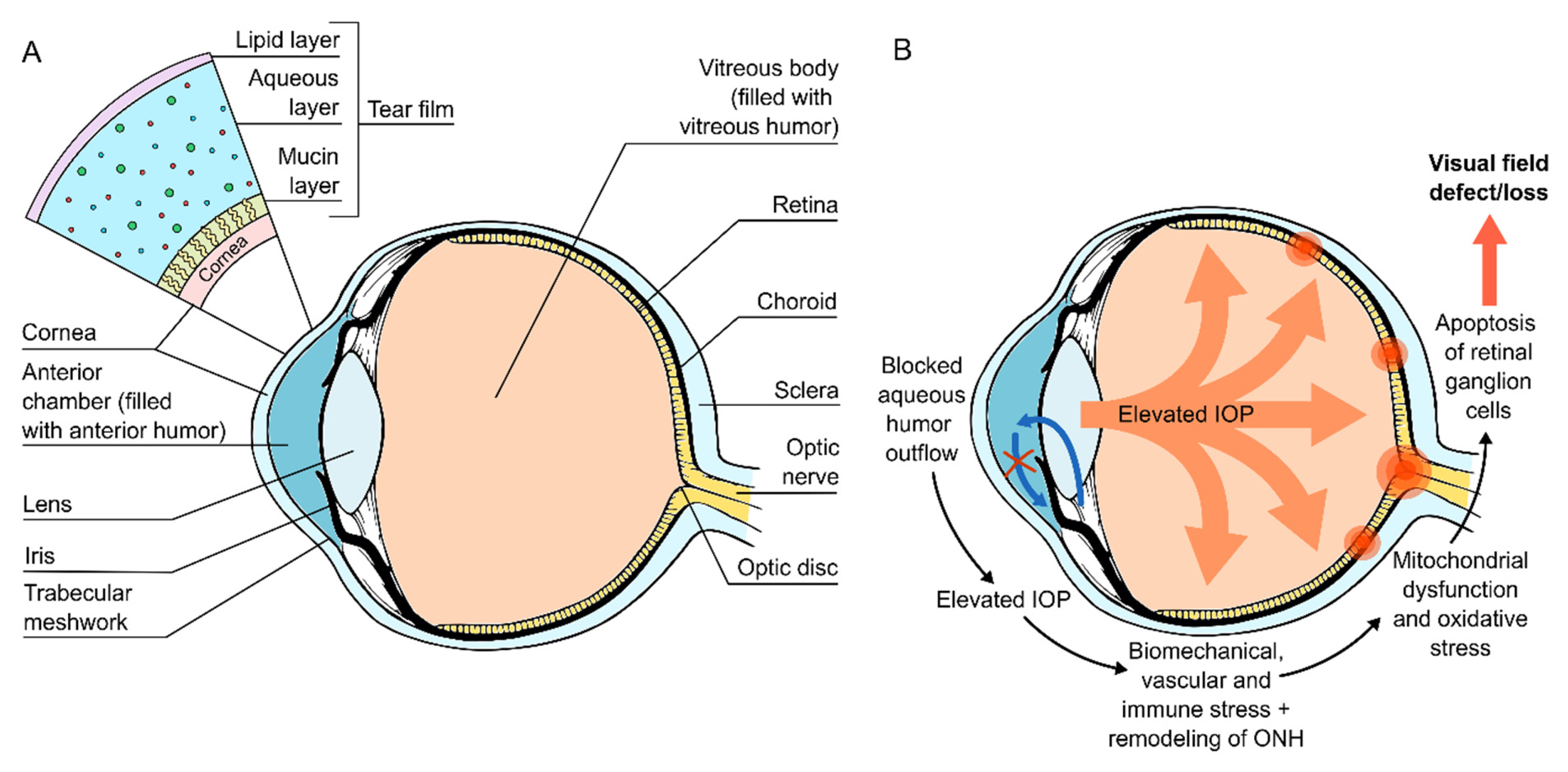
Figure 1. Fluid in the eye is drained through an intricate system and ineffective drainage of aqueous humour results in a backlog of fluid accumulation within the drainage system of the eye. This then causes direct damage to optic nerve, in addition to its blood supply 8.
Glaucoma is asymptomatic, is it possible to identify those at risk of the disease early? Since we already know that raised IOP is a major risk factor in glaucoma pathogenesis and progression, we can perhaps monitor the IOP regularly to identify those at risk. It is important to note that IOP changes with posture and diurnal variation 9. For instance, emerging studies have shown IOP being higher at night compared to the day, particularly in supine position 10. Therefore, glaucoma patients often require 24 hours IOP monitoring on a daily basis. However, none of the currently available ocular tonometers are considered effective in continuous monitoring of IOP outside the clinical setting. In addition, glaucoma patients often require regular clinic visits for monitoring disease progression, but such practices are often plaque by patient compliance and the recent COVID-19 pandemic 11. To overcome this issue, there are commercially available portal handheld ocular tonometers that enable patients to monitor IOP at home, but again their use overnight remains limited, especially during sleep 12.
Is there any solution to this problem? Well, kind of, as emerging studies on wearable ocular tonometers, such as the TriggerFish lens, allow continuous monitoring of IOP in both clinics and at home. Yet, their long-term use remains impractical due to their side effects. These devices can cause eye pain, superficial punctate keratitis, corneal epithelial defects, and conjunctival erythema 13. So, what then? Very recent studies on smart soft contact lenses (SSCL) highlighted the use of highly soft, thin, stretchable ocular tonometers integrated contact lenses for glaucoma monitoring. You may then ask, what is so special about them? These lenses are more comfortable, provides a clear vision, and can be worn overnight for continuous IOP monitoring, even during sleep 14. In addition, they are durable, with drug delivering capabilities on-demand, thereby allowing self-monitoring 15.
Continuous monitoring of IOP, particularly during sleep, remains the greatest challenge in glaucoma care, and SSCL seems to be the way forward. The SSCLs are developed from the normal soft contact lenses that are used daily without altering their intrinsic properties such as lens power, softness, transparency, wettability, oxygen transmissibility, and overnight wearability. Furthermore, both animal and human studies have demonstrated SSCL as user-friendly since these can be easily fit across different corneal curvatures and thicknesses 14. Moreover, the results of SSCLs are promising even in their experimental phase, and remain a viable option in the management of glaucoma.
Recently, a team led by Professor Sei Kwang Hahn of the Pohang University of Science and Technology managed to develop a wireless theranostic smart contact lens that was used to monitor IOP in glaucoma-induced rabbits, and deliver timolol (beta-blocker) upon sensing a change in IOP pressure through electrical triggers 15. How does autonomous timolol delivery work in SSCL? The IOP-lowering drug such as timolol was loaded into the flexible drug delivery system (DDS) with a biocompatible protection layer that was able to release timolol from the reservoirs through an electrochemical reaction of gold channels during phases of high IOP as illustrated in Figure 2 15. The study concluded that the theranostic smart contact lens was able to reduce the IOP among glaucoma-induced rabbits even at different IOP levels. Furthermore, the progression of the disease was successfully inhibited, with a well-preserved structure of retina achieved among these rabbits 15. Even though this type of treatment is still in early experimental stage, it opens new avenues allowing personalised glaucoma treatment. In conclusion, glaucoma is a silent, asymptomatic disease which requires early monitoring and aggressive treatment to prevent vision loss among sufferers.
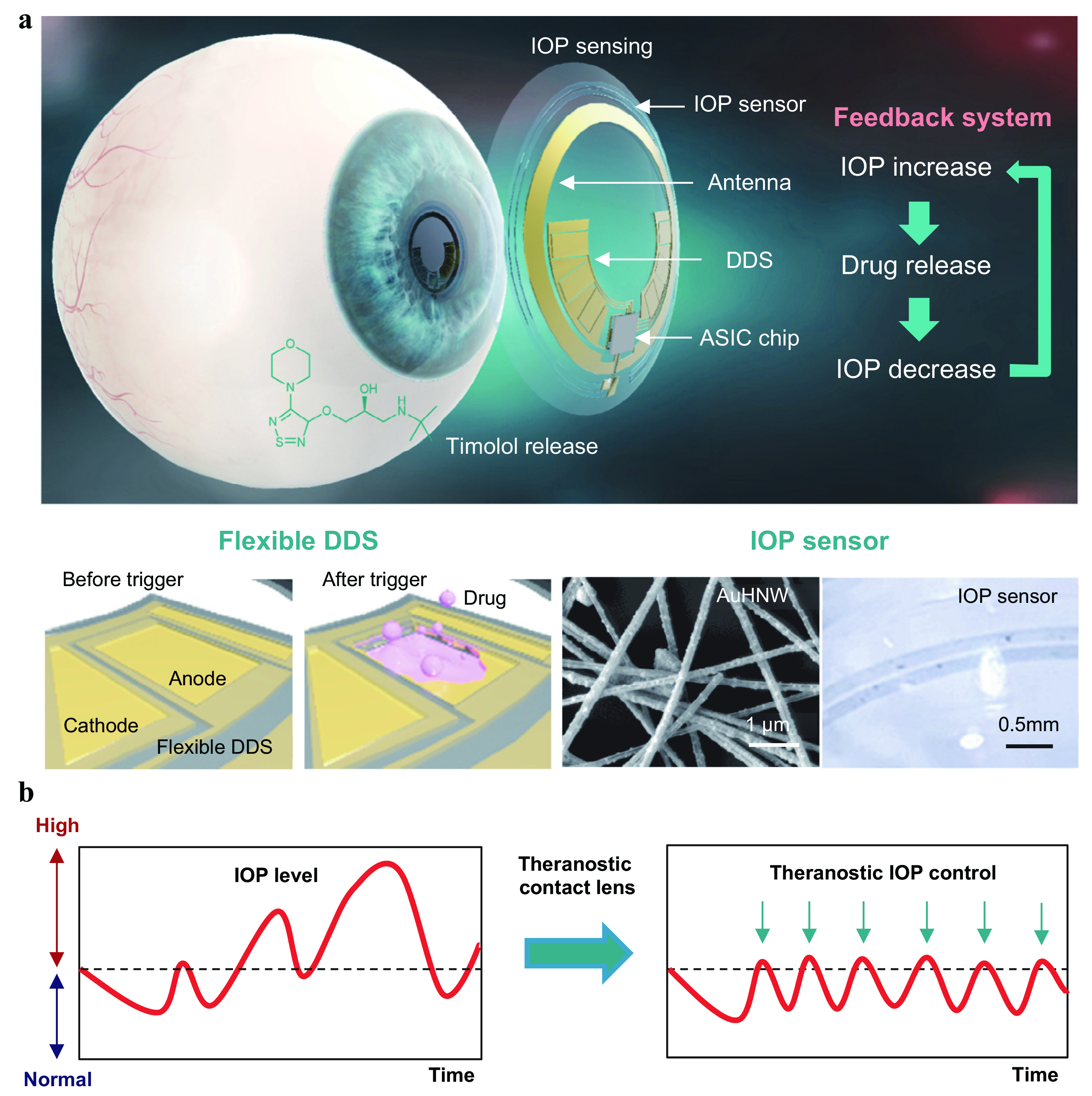
Figure 2. Shows the basic concept of the smart contact lens and the way IOP is monitored, followed by Timolol release through the flexible drug delivery system (DDS) with dissolution of the gold-plates 15.
References
1. Cook C, et al,. Can J Ophthalmol 2012; 47(3): 223-6. 2. Jonas JB, et al., Lancet 2017; 390(10108): 2183-93. 3. Song P, et al., National and subnational prevalence and burden of glaucoma in China: A systematic analysis. J Glob Health 2017; 7(2): 020705. 4. Schuster AK, et al., Dtsch Arztebl Int 2020; 117(13): 225-34. 5. Halawa OA, et al., Am J Ophthalmol 2022; 242: 69-76. 6. Kang JH, et al.,. Transl Vis Sci Technol 2022; 11(7): 21. 7. Dietze J, et al., Glaucoma. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2022. 8. Nättinen J, et., Int J Mol Sci 2022; 23(15). 9. Gautam N, et al., Br J Ophthalmol 2016; 100(4): 537-41. 10. Flatau A, et al., JAMA Ophthalmol 2016; 134(4): 375-82. 11. Mostafa I, et al., Eye (Lond) 2021; 35(2): 448-54. 12. Pronin S, et al., JAMA Ophthalmol 2017; 135(10): 1030-6. 13. Vitish-Sharma P, et al., Acta Ophthalmol 2018; 96(2): e242-e6. 14. Zhang J, et al., Nat Commun 2022; 13(1): 5518. 15. Kim TY, et al., Nat Commun 2022; 13(1): 6801.





