
Clostridioides difficile infection (CDI) is a common nosocomial disease, which leads to symptoms ranging from mild diarrhoea to life-threatening colitis and toxic megacolon1. The estimated local crude incidence rate of CDI among adults aged ≥65 years was 133 to 207 cases per 100,000 population, with an increasing trend observed from 2006 to 20142. Notably, the domination of Clostridioides difficile in the gut starts with the disruption of the balance in the gut microbiome3. Intestinal microbiome restoration is thus considered a treatment strategy against CDI. Thanks to the contribution of investigators, clinicians and patients, the first-in-class microbiota-based therapy (MBT) indicated for the prevention of recurrence of CDI in adults following antibiotic treatment has recently been approved by the U.S. Food and Drug Administration (FDA)4. The approval of MBT highlights a significant breakthrough in the treatment of recurrent CDI as well as dysbiosis-associated diseases.
The human gut microbiome plays a key role in regulating metabolic and immune homeostasis and serves as a mediator for resisting certain pathogens5, whereas disruption to the normal composition of the gut microbiome, commonly referred to as dysbiosis, would potentially result in pathologic outcomes. Among dysbiosis-associated diseases, CDI is associated with significant morbidity and mortality, particularly for patients over 75 years of age1.
A recent territory-wide survey by Guo et al (2021) involving 17,105 local cases of CDI reported that 91.9% of CDI were healthcare-associated and 6.0% were community-associated (Figure 1). Essentially, the 60-day recurrence rate of CDI was about 11%, whereas the 30-day mortality rate in 2019 reached 16.8%. Of note, the results also indicated a significant correlation between CDI incidence trend and the overall antimicrobial drug use (r = 0.865, p <0.001)1.
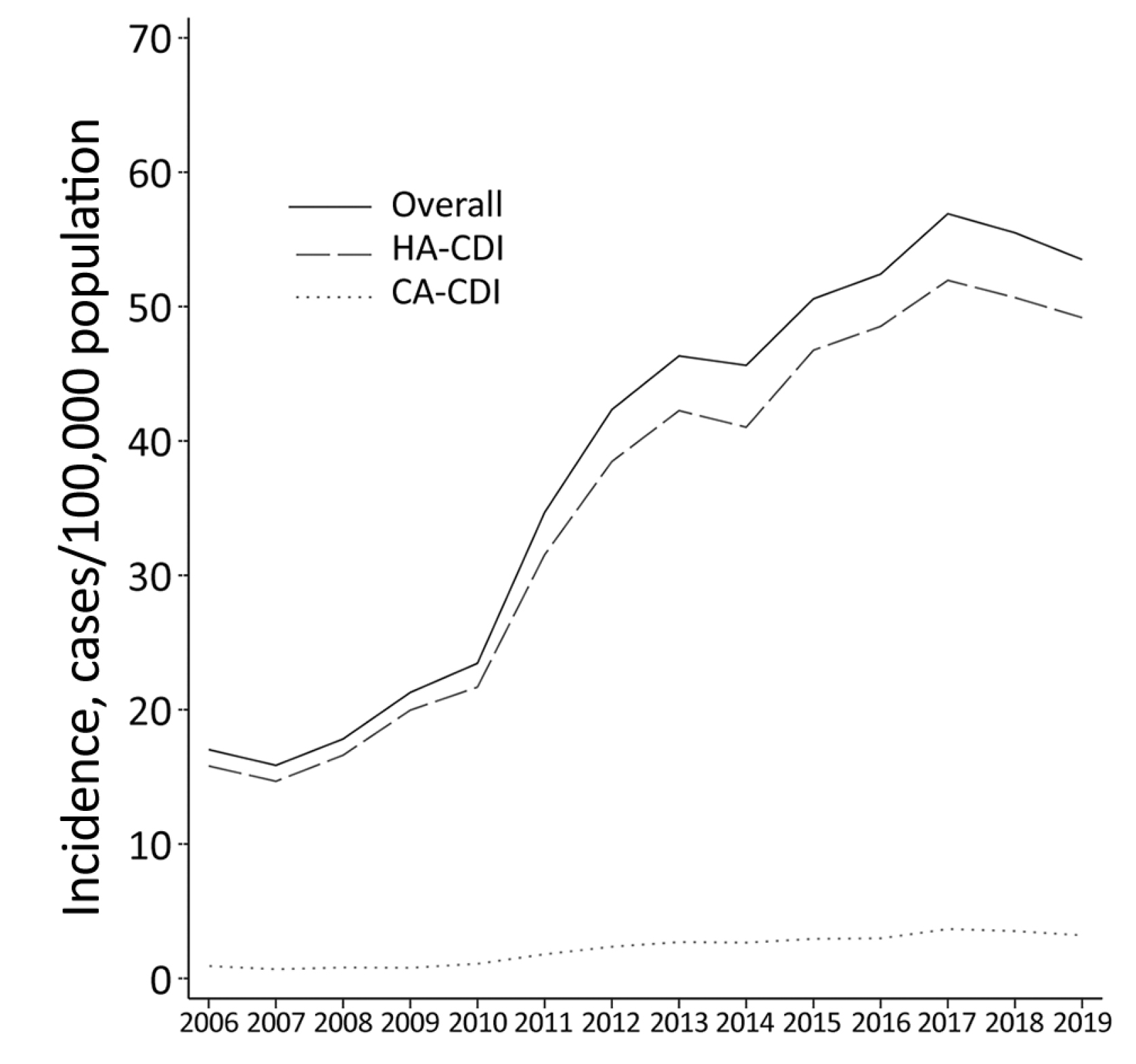
Figure 1 : Local CDI in adults in 2006-20191, HA: healthcare-associated, CA: community-associated
The observations in the survey agreed with the existing literature suggesting the increased risk of CDI upon antibiotic treatment6. Apart from the lack of nutrients, Clostridioides difficile can tolerate unfavourable conditions, such as heat and acidic environment7. Upon exposure to antibiotics, the production of spores allows Clostridioides difficile to survive in the host8. While most gut microbes are suppressed by antibiotics, the disturbed balance in the gut microbiome then offers an ideal environment nurturing recurrent CDI.
Interestingly, as shown in Figure 1, the local trend of CDI in 2018-2019 exhibited a downturn. This decrease may be explained by the initiation of an antibiotic stewardship program initiated in 20171. In this regard, optimising the pattern of antibiotic use would help contain the disease burden of CDI macroscopically.
Yet, for individual patients with recurrent CDI, efficacious and safe therapeutics controlling the disease and normalising gut microbiome is vital. In particular, the idea of faecal microbiota transplantation (FMT) is to restore the balance in the gut microbiome by transplanting gut microbiota extracted from healthy donor stool to the patient. Thus, stringent criteria for donor selection and screening tests are required to ensure patient safety. During transplantation, the FMT product was delivered either via a feeding tube or through scope channels during oesophagogastroduodenoscopy (OGD) or colonoscopy2. The local retrospective study by Lui et al (2019) involving 24 patients with recurrent or refractory CDI demonstrated that resolution of diarrhoea without relapse within 8 weeks after FMT was achieved in 87.5% of the patients. The FMT treatment was well-tolerated, and no serious adverse events attributable to FMT were reported2.
While FMT has been demonstrated to effectively control symptoms of CDI, limitations hinder its application in clinical practice. For instance, as stringent criteria for donor selection are required, finding suitable donors for FMT is challenging in reality. In the trial by Paramsothy et al (2015), the proportion of eligible donors for FMT was as low as only 10%9. Besides, the potential risk of transmitting antibiotic-resistant genes (ARGs) derived from donor stool samples is also a clinical concern.
The development of MBT provides a viable solution to overcoming the clinical difficulty associated with conventional FMT. While MBT is designed to mimic FMT, the therapeutic is prepared from stool samples donated by qualified individuals using standardised, quality-controlled processes. Patients do not have to find their own donor or wait for the results of screening tests. Remarkably, the MBT is supplied in a ready-to-use enema format10.
The efficacy and safety profile of the MBT in preventing recurrent CDI were evaluated in the Phase III PUNCH CD3 trial. In the trial, 262 patients with documentation of recurrent CDI were randomly allocated to receive either MBT or placebo. The primary endpoint was the absence of CDI diarrhoea within 8 weeks after completing the study treatment. The model-estimated treatment success rate at 8 weeks for MBT was 70.6% versus 57.5% for placebo (Figure 2). Essentially, more than 90% of the participants who achieved treatment success at 8 weeks had sustained responses through 6 months in both MBT and the placebo groups. Moreover, the results suggested that MBT was well-tolerated, with manageable adverse events11.
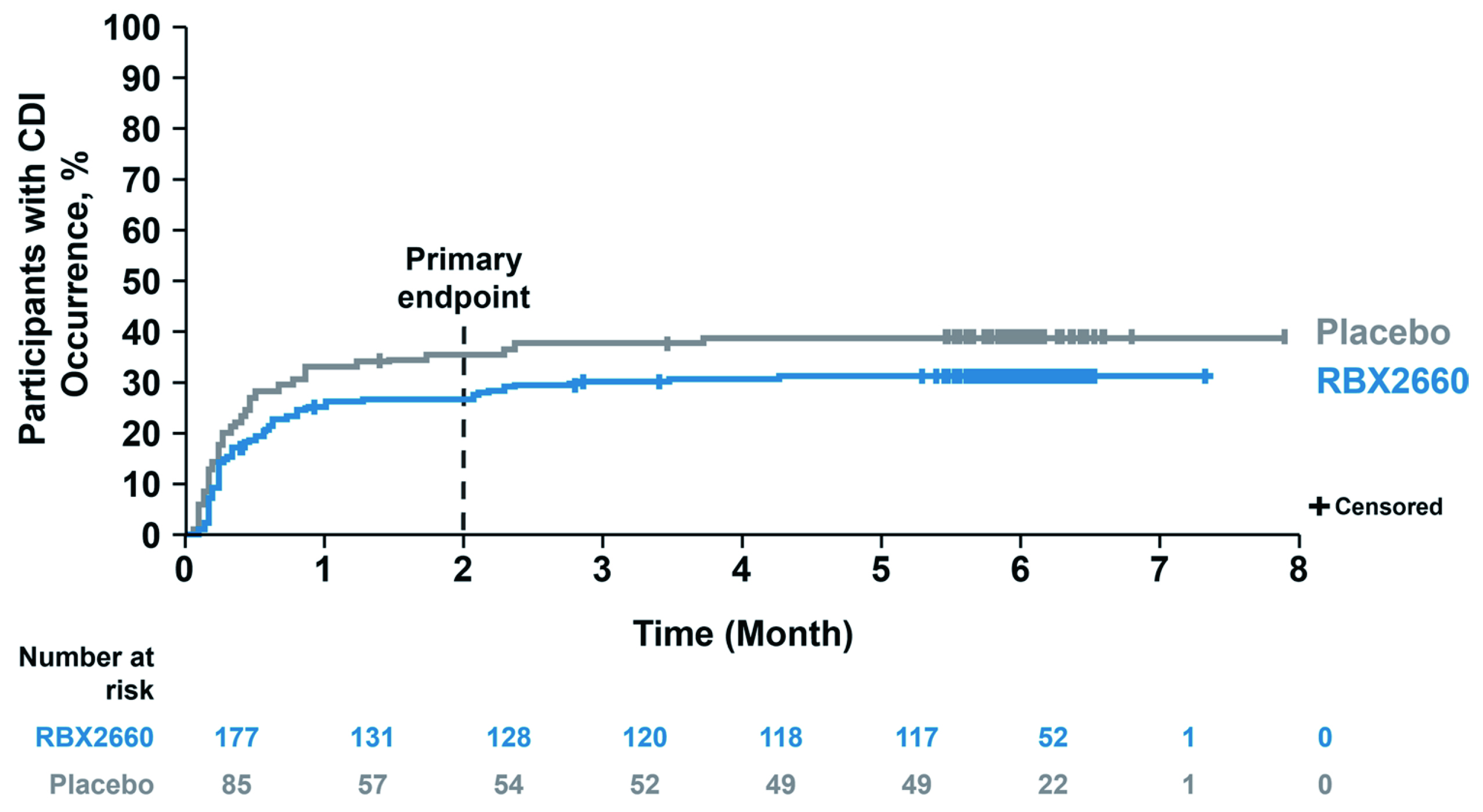
Figure 2 : Time to CDI recurrence through 6 months in PUNCH CD3 trial11
The results of the PUNCH CD3 trial showed that MBT is a safe and effective treatment to reduce recurrent CDI following standard-of-care antibiotics with a sustained response through 6 months. Based on the promising results, the MBT was approved by the FDA as the first-in-class therapeutic targeting recurrent CDI in adults following antibiotic treatment4. The thumbs-up from the FDA to MBT undoubtedly is a milestone in the microbiome field. While only limited treatment options were available before the FDA approval, the MBT brings hope to patients living with the devastating cycle of recurrent CDI and debilitating symptoms.
References
1. Guo et al. Emerg Infect Dis 2021; 27: 3036. 2. Lui et al. Hong Kong Med J 2019; 25: 178–82. 3. Czepie et al. Eur J Clin Microbiol Infect Dis 2019; 38: 1211. 4. FDA.FDA Approves First Fecal Microbiota Product. 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product. 5. Young. BMJ 2017; 356. DOI:10.1136/BMJ.J831. 6. Hensgens et al. J Antimicrob Chemother 2012; 67: 742–8. 7. Setlow. J Appl Microbiol 2006; 101: 514–25. 8. Britton et al. Gastroenterology 2014; 146: 1547. 9. Paramsothy et al. Inflamm Bowel Dis 2015; 21: 1600–6. 10. Tracey et al. SGNA 2015. 11. Khanna et al. Drugs 2022; 82. DOI:10.1007/S40265-022-01797-X.





