Chronic hepatitis B (CHB) is a well-documented cause of liver failure and hepatocellular carcinoma (HCC). While long-term nucleos(t)ide analogues (NAs) treatment has been shown to be effective in the suppression of HBV replication, entecavir (ETV) and tenofovir alafenamide (TAF) are recommended over tenofovir disoproxil fumarate (TDF) by virtue of their lower nephrotoxicity1. Besides, in addition to effective suppression of hepatitis B virus (HBV), recent clinical data demonstrated that TAF treatment was associated with a lower risk of chronic kidney disease (CKD) progression than ETV2. These findings highlighted that TAF treatment would effectively suppress HBV viral load and is associated with lower rates of renal abnormalities.
Recommended First-line Treatment for CHB
Since the publication of the 2016 AASLD Hepatitis B Guideline, TAF has been advocated as a preferred HBV therapy, along with ETV and TDF3. Like TDF, TAF inhibits reverse transcription of pre-genomic RNA to HBV DNA. Nonetheless, TAF has higher plasma stability than TDF, enabling more efficient delivery of the active metabolite tenofovir diphosphate (TFV) intracellularly at much lower doses. Given that circulating plasma TFV level is associated with renal and bone toxicity, the lower therapeutic dose of TAF thus reduces systemic exposure and hence decreases renal and bone toxicity4.
The improved safety achieved by TAF over TDF in CHB patients was confirmed in the Phase III trial by Lampertico etal (2020). In the trial, CHB patients on TDF treatment for 48 months or more were randomly assigned to receive TAF or continue TDF treatment. At week 48, TAF treatment (n = 243) was shown to have non-inferior efficacy in HBV suppression to TDF (n = 245). Essentially, patients on TAF had significantly increased bone mineral density at the hip and spine (p <0.0001 for both) and creatinine clearance (p <0.0001) relative to TDF5. These findings suggested that TAF would be a preferred alternative of TDF for CHB patients with comparable efficacy in viral suppression and improved safety.
Preferred Renal Safety Profile
While ETV and TAF are the preferred agents for treating CHB, the renal safety of the treatments is an important issue considering that the two drugs could have different renal effects due to their specific mechanisms of drug metabolism. Hence, a recent clinical study was conducted by Jung et al (2022) to compare the risk of kidney function decline among CHB patients treated with ETV or TAF. In the study, chronic kidney disease (CKD) progression was defined as an increase in CKD stage by at least one stage for at least three consecutive months during follow- up, whereas CKD stages were estimated based on the calculated eGFR and classified according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria2.
Based on a 1:1 propensity score matching in the cohort of 1,988 CHB patients from a University Health System, 149 CHB patients were identified in each treatment group, with the baseline covariates closely balanced. In the matched cohort, 61 patients had developed CKD progression. Of which, 47 patients were treated with ETV (19.9 per 1,000 person-years) and 14 were TAF- treated (5.1 per 1,000 person-years, p <0.001, Figure 1)2. Besides, the risk of CKD progression was significantly higher in patients on ETV relative to those TAF-treated (adjusted hazard ratio [HR]: 4.05, p <0.001)2. Particularly, at 36 months, the degrees of increase in serum creatinine (p = 0.034, Figure 2) and decrease in eGFR (p = 0.01, Figure 3) in ETV group were significantly greater as compared to TAF group2. These results reflected the real-world impacts of ETV and TAF on renal safety among CHB patients that ETV treatment was associated with a higher risk of decline in kidney function as compared to TAF.
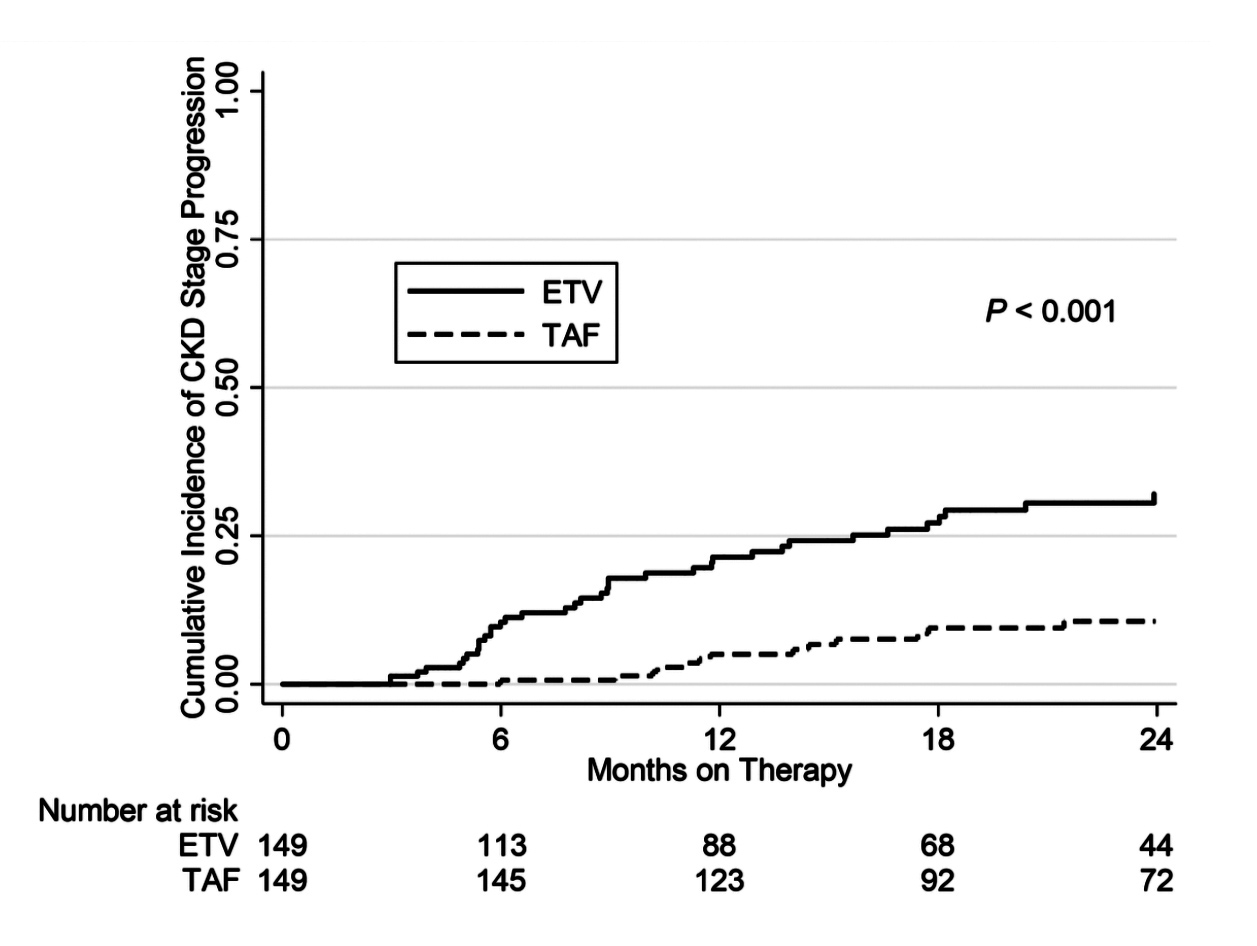
Figure 1.Cumulative incidence of progression in CKD stage ≥12
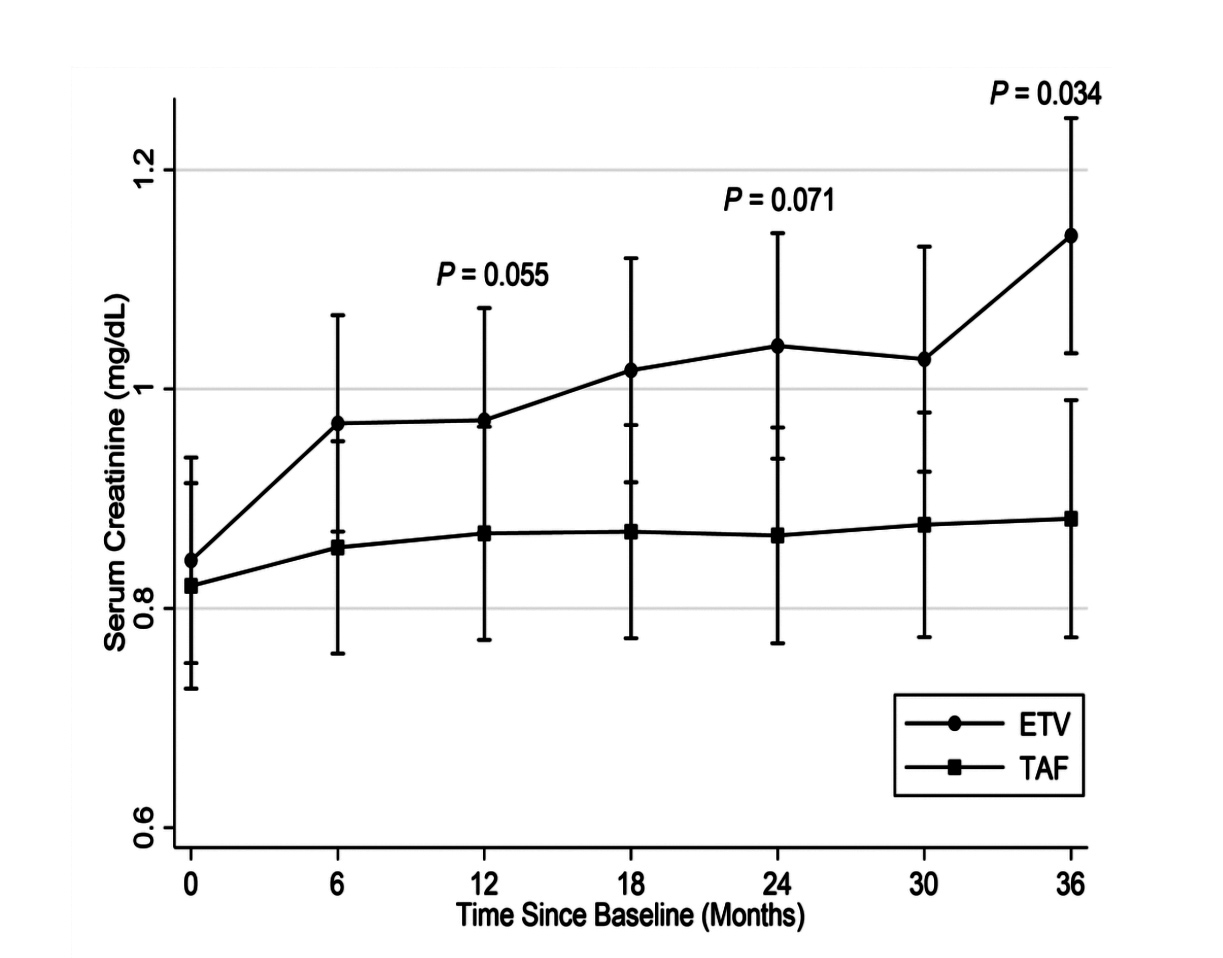
Figure 2.Changes in serum creatinine2 ≥12
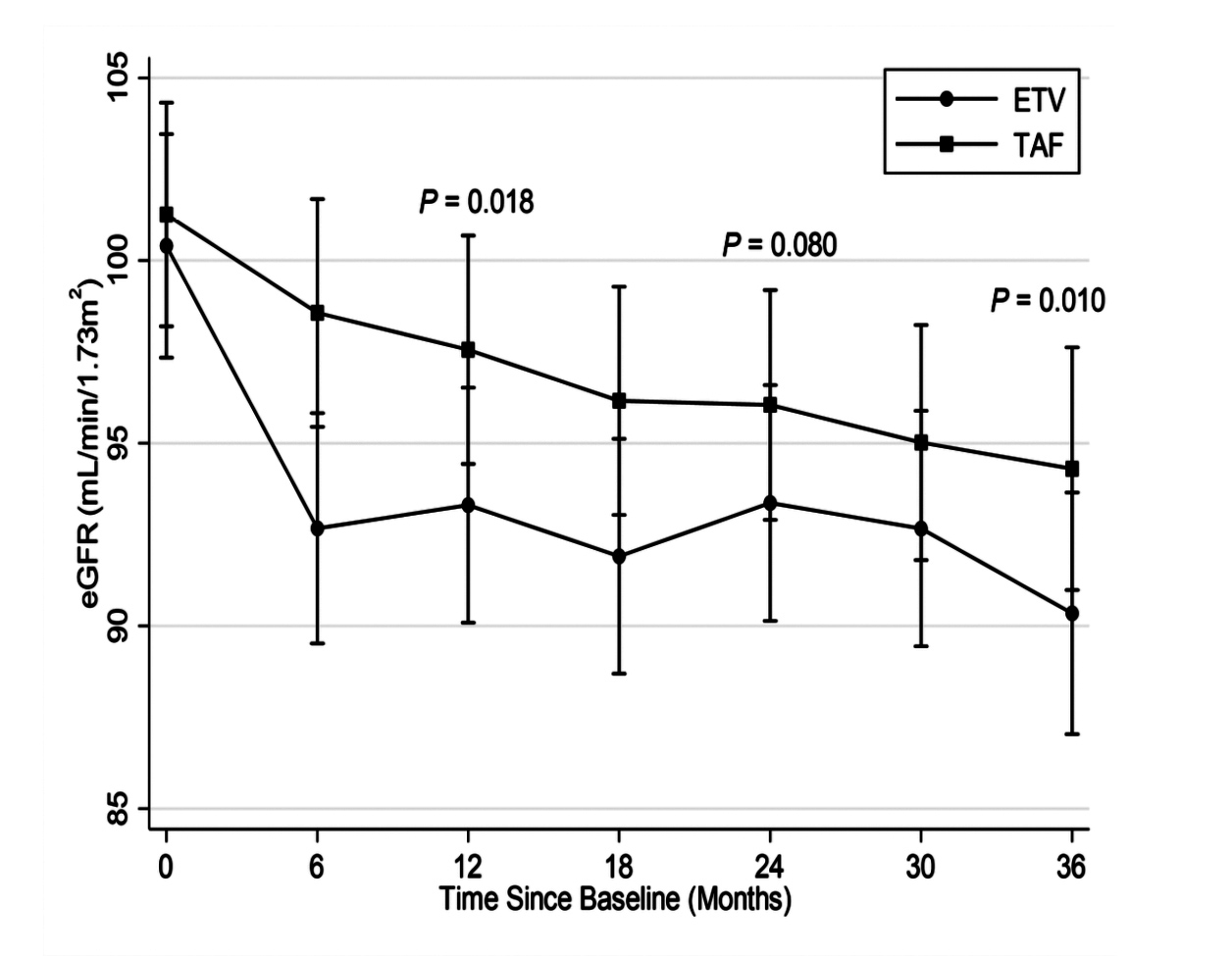
Figure 3.Changes in eGFR2 ≥12

Figure 4.Change in proportion of patients achieved both HBV DNA suppression and ALT normalisation (NALT) at 48 weeks after switching from ETV to TAF1 ≥12
Clinical Benefits of Switching from ETV to TAF
Owing to the increased risk of renal abnormalities of ETV relative to TAF, it would be reasonable to consider the switching from ETV to TAF. A retrospective cohort study by Ogawa et al (2019), which included 313 CHB patients who switched to TAF monotherapy after treatment with ETV (n = 191) or a NA combination(n = 122) for over two years. According to the laboratory responses evaluated at 48 weeks after switching therapy, a significant increase in HBV DNA suppression rate, from 75.9% to 96.9% (P <0.001), was observed in prior ETV group. Moreover, the proportion of patients who achieved both HBV DNA suppression (<20 IU/mL) and alanine aminotransferase (ALT) normalisation by the AASLD criteria was significantly increased from 55.5% to 73.8% (p <0.001, Figure 4)1.
The results further indicated that no patient developed significant renal glomerular or proximal tubular dysfunction following the switch to TAF. Of importance, a slight increase in eGFR was observed in the CKD (<60 mL/min/1.73m2) patient group (+0.40 mL/min/1.73m2), which was significantly improved as compared to the non-CKD group (p = 0.001)1. Moreover, it is noteworthy that no TAF dose adjustment is required for patients with renal impairment, except for end-stage renal failure without hemodialysis, whereas dose adjustment of ETV is required for patients with creatinine clearance <50 mL/min because ETV is excreted mainly by the kidneys1.
The improvement in renal function and bone metabolism upon switching from ETV to TAF was further demonstrated in a study by Notsumata et al (2020). In the study, the changes in biomarkers of renal tubular dysfunction, urinary L-FABP level, and bone metabolism, FGF23 level, in 38 CHB patients were evaluated. The results indicated a significant decrease in urinary L-FABP level from 4.32 to 2.96 at 4 weeks after the switch (p = 0.039), which suggested recovery of renal tubular damage. Besides, a significant increase in FGF23 level was observed from 34.7 to 42.5 at 12 weeks after the switch, which implied an improvement in hormone regulation of phosphorus metabolism6. Thus, the changes in biomarkers suggested that switching treatment from ETV to TAF would improve renal function and bone metabolism in CHB patients.
Optimising CHB Treatment Outcomes
CHB is a global health burden associated with the development of cirrhotic complications and HCC, affecting millions of people worldwide. This highlights the clinical importance of antiviral therapy suppressing HBV. However, treatment-related side effects of CHB treatments have been a major concern. While TDF has been documented to be associated with renal and bone toxicity, ETV and TAF are advocated as the preferred CHB therapies. Provided the higher prevalence of CKD among CHB patients compared to the general population and that lifelong treatment is prescribed for most of the patients, the renal safety profile of CHB therapy is thus an important consideration.
Recent real-world data revealed that ETV was associated with an increased risk of kidney function decline relative to TAF in CHB patients. Whereas TAF effectively suppresses HBV viral load without compromising renal safety. Moreover, switching therapy from ETV to TAF would likely yield improved HBV suppression as well as renal and bone safety. Thus, TAF would be the preferred therapy for long-term management of CHB, particularly in patients with CKD.
References
1. Ogawa et al. Liver Int 2020; 40: 1578-89. 2. Jung et al. Liver Int 2022; published online March 7. DOI:10.1111/LIV.15208. 3. Terrault et al. Hepatology 2018; 67: 1560-99. 4. Byrne et al. Therap Adv Gastroenterol 2018; 11. DOI:10.1177/1756284818786108. 5. Lampertico et al. Lancet Gastroenterol Hepatol 2020; 5: 441-53. 6. Notsumata et al. Hepatol Res 2020; 50: 402-4.





