
Evaluating Efficacy of Anti-migraine Medications with the Capsaicin-induced Dermal Vasodilation Model
Migraine is a common disabling disorder accounting for more than half of the disabilities attributable to all neurological diseases1. It manifests as pain with associated sensory disturbances and is considered as a chronic condition with episodic manifestations, but the exact neurobiological mechanisms leading to migraine pain are not fully understood. Recent studies suggest that migraine pain originates from the activation of trigeminal nerve terminals in meninges followed by neuronal sensitisation via the release of migraine mediators2. In particular, considerable evidence implicates calcitonin gene-related peptide (CGRP) in migraine pathophysiology and thus making it a pharmacological target for the treatment of migraine pain. Nonetheless, practical tests for monitoring the release of CGRP are needed to evaluate the efficacy of the therapeutics. This article reviews the practical monitoring of migraine pain based on evaluating CGRP blocking with the capsaicin-induced dermal vasodilation (CIDV) model.
CGRP as a Mediator of Migraine Pain
CGRP, a 37-amino acid neuropeptide, and its receptor are expressed in unmyelinated C-fibres, myelinated Aδ fibres and in multiple regions in the central nervous system. It was first described as a potent vasodilator acting primarily on vascular smooth muscle cells (VSMCs)3. Upon interacting with VSMCs, CGRP would trigger local redness and warmth and limit swelling as well as allodynia. This is collectively known as neurogenic inflammation4, and as a result participates in peripheral sensitisation.
Apart from vasodilation, CGRP is suggested to play a role in the pathogenesis of migraine, which was originally ascribed to its vasodilating action at the level of the intracranial arteries5. In recent years, CGRP has been shown to modulate neuronal excitability, particularly facilitating pain response in the trigeminal system and trigeminal nucleus caudalis (TNc)6. The association between CGRP and migraine pain has been previously demonstrated. For instance, stimulation of the trigeminal ganglion in humans and cats trigger CGRP release7, whereas serum CGRP levels were elevated during attacks in episodic8 and chronic migraine9. Hence, biologics that work against CGRP or its receptor, have been developed for treating and/or preventing migraine pain.
Monitoring Potency of Anti-migraine Drug with the CIDV Model
Capsaicin is a vanilloid responsible for the pungent taste of chili peppers. It has been used extensively in human pain models to induce experimental pain. When capsaicin is applied topically onto human skin, it rapidly triggers local neurogenic inflammation by binding to the transient receptor potential vanilloid type 1 (TRPV1) receptor present on sensory nerve endings of the skin. Of note, former evidence indicates that the release of CGRP is a major initiator of this response (Figure 1)10. Hence, the novel in vivo non-invasion pharmacodynamic assay, i.e. the CIDV model, for measuring blockage of CGRP has been developed. In the CIDV model, TRPV1 receptors at peripheral sensory nerves are activated by topically applied capsaicin followed by neurogenic inflammation, which is characterised by local vasodilation. The increased dermal blood flow (DBF), which is mediated mainly by CGRP, can then be measured using laser Doppler imaging (Figure 2). Essentially, the increased DBF can be significantly blocked by CGRP receptor antagonists, thus permitting the assessment of antagonist potency in vivo against endogenously released CGRP11.
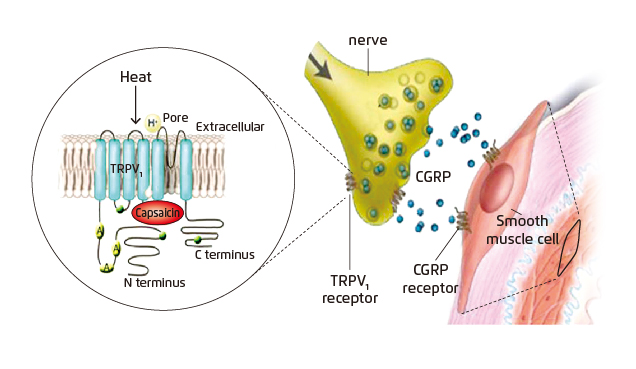
Figure 1. Activation of TRPV1 receptor by capsaicin10
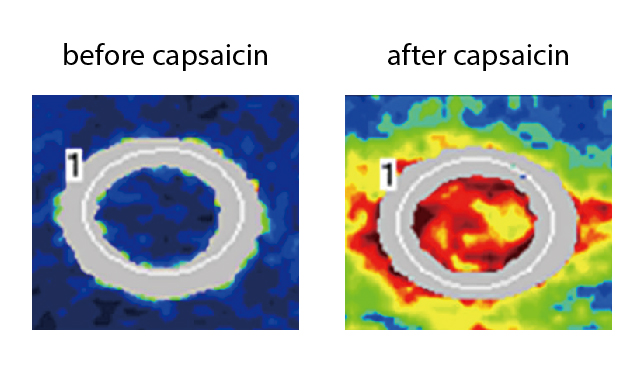
Figure 2. Capsaicin-induced increased DBF measured with laser Doppler imaging11
CIDV in Action
The application of CIDV model for evaluating the CGRP blocking capacity, and hence anti-migraine efficacies, of potential therapeutics has been demonstrated in previous trials. Briefly, the candidate drug was provided to the human subjects followed by a single topical dose of capsaicin on the volar surface of the subjects’ forearms (Figure 3). Laser Doppler scans were conducted immediately before capsaicin application (baseline) and at certain time intervals, e.g. every 30 minutes, allowing the maximal increase in DBF to coincide with the appropriate time points. In addition, subjects’ plasma samples were collected immediately after drug intake and laser Doppler DBF measurement for pharmacokinetic/pharmacodynamic (PK/PD) analysis. By varying the dosage of the candidate drug, the association between drug dosage and DBF could thus be demonstrated (Figure 4)12.
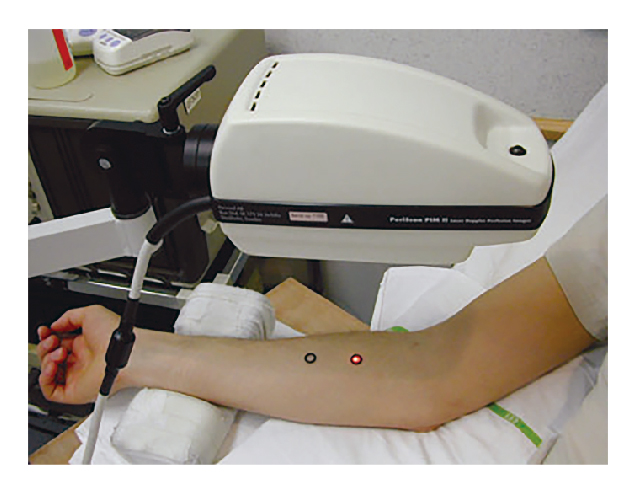
Figure 3. Clinical assessment of DBF using laser Doppler12
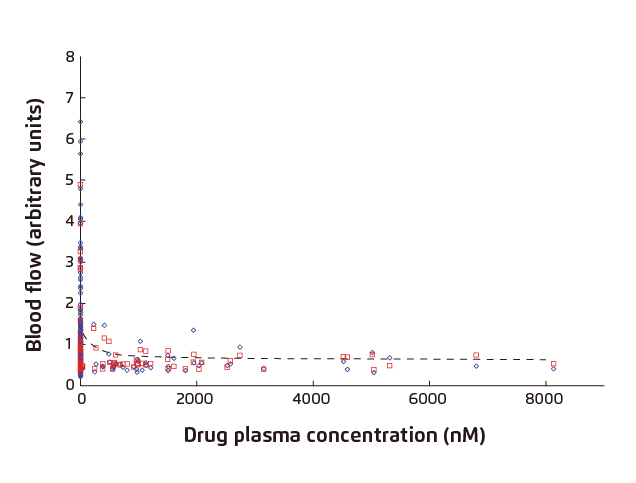
Figure 4. DBF vs. plasma concentration of candidate drug
(◊: Actual Flow; □: Individual Predicted; ---: Population Predicted)12
Further Optimisation on the CIDV Model
The CIDV model is an in vivo pharmacodynamic model, which is non-invasive and has a rapid and objective endpoint. The model is repeatable and hence allows early evaluation of efficacies of CGRP inhibitors in reversing neurogenic inflammation. However, the described CIDV model reflects only drugs’ efficacy in peripheral DBF while the information might not be predictive for the changes in the cranial circulation such as the trigeminovascular system4. Nonetheless, Ibrahimi et al (2014) developed an optimised CIDV model overcoming the limitation, by performing the test on the forehead instead of the forearm13. The optimised model was applied in a subsequent clinical trial evaluating the anti-migraine efficacy of sumatriptan14.
Although there are numerous works suggesting the crucial role played by CGRP in the onset of migraines, further evidence confirming the specificity of peripheral CGRP levels as the biomarker for migraine is required. Essentially, solid evidence on the specificity of peripheral CGRP level resembling migraine would improve the confidence in information generated by the CIDV model and, hence, allow evaluation of potential therapeutics at the earliest possible stage of drug development.
References
1. Murray et al. Lancet. 2012;380(9859):2197-2223. 2. Levy. Headache J Head Face Pain. 2010;50(5):909-916. 3. Raddant et al. Expert Rev Mol Med. 13:e36. 4. Buntinx et al. Br J Clin Pharmacol. 2015;80(5):992. 5. Brain et al. Physiol Rev. 2004;84(3):903-934. 6. Giamberardino et al. J Pain Res. 2017;10:2751. 7. Goadsby et al. Ann Neurol. 1993;33(1):48-56. 8. Ashina et al. Pain. 2000;86(1):133-138. 9. Cernuda-Morollon et al. Neurology. 2013;81(14):1191-1196. 10. Hershey et al. Regul Pept. 2005;127(1-3):71-77. 11. Van der Schueren et al. J Pharmacol Exp Ther. 2008;325(1):248-255. 12. Sinclair et al. Br J Clin Pharmacol. 2010;69(1):15. 13. Ibrahimi et al. Cephalalgia. 2014;34(7):514-522. 14. Ibrahimi et al. Cephalalgia. 2017;37(1):94-98.





