Injection with botulinum toxin type A (BoNT-A) remains one of the most common treatments for both males and females. Interestingly, since 2021, there had been 6.2 million cosmetic procedures performed with BoNT-A and staggering 3.1 million of these had been performed in those aged 35-50 years, mostly females1. However, recent studies have raised concerns since repeated injections may cause undesirable and unanticipated muscle atrophy that may become irreversible2. Furthermore, the potential for BoNT-A immunoresistance and secondary nonresponse related to neutralising antibodies (NAbs) formation resulted in expansion of BoNT-A range products3. Among these is the newly Food and Drug Administration (FDA) approved letibotulinumtoxin A, which has shown not only a higher efficacy and safety, instead it has also shown to improve psychosocial burden associated with glabellar lines across different patient groups4.
The use of BoNT-A in aesthetic medicine has increased markedly since the first application in this setting during the mid-1980s. Currently, aesthetic use of BoNT-A include the treatment of glabellar line, forehead wrinkles, periorbital and perioral lines, platysmal bands, horizontal neck lines, and the masseter applications. The American Society of Plastic Surgeons reported around 15.4 million minimally invasive aesthetic procedures performed in the US since 2016, and 7 million US dollars (USD) was being spent on BoNT-A related procedures5. Presently, onabotuliniumtoxin A (ONA) abobotulinumtoxin A (ABO) and incobutulinumtoxin A (INCO) are the most widely used BoNT-A formulations and all three are FDA approved for medical and aesthetic indications. Comparative clinical trials indicated that ONA, INCO, and ABO have similar efficacy for aesthetic application6. However, due to the pharmacologically unnecessary impurities contained in some preparation of BoNT-A, there is an increased risk of mounting adaptive immune response and production of NAbs against BoNT-A and other injected proteins7.
Furthermore, even though INCO formulation has been reported to have no NAbs-related secondary non-response (SNR), studies have shown patients treated with INCO reported diminishing efficacy which was likely related to factors other than NAbs, such as incorrect injection technique7. In addition, BoNT-A related has also shown to induce muscle atrophy with fibrosis, and necrosis in both animal and human subjects. Moreover, a significant muscle balance reduction of 18% to 60% after single or seriated BoNT-A injections were observed in 9 out of 10 animal studies. But are there any genetic alterations induced by the BoNT-A injections? A study by Nassif et al., (2022) suggested that BoNT-A injections may cause genetic alterations that results in muscle atrophy. The study found that a single injection of BoNT-A may actually induce molecular changes that may produce short- or long-term effects8. In addition, the risk of mild to moderate dysphagia has been reported with even conservative doses of ABO formulations9.
With the advancement of living standards in modern society, and the emergence of an aging population, an increasing number of individuals are seeking for anti-aging treatment. Therefore, in the field of cosmetics, the market share for anti-aging products is increasing year by year10. At the same time, the appearance of accentuated vertical glabellar lines, caused by activity of procerus and bilateral corrugator muscles, can cause a depressed, angry or fatigue look. In fact, recent studies have shown that muscular activities of procerus and corrugator muscles increases with age, reinforcing the idea that the formation of both static and dynamic glabellar folds is caused by hyperactivity of these muscles. To decrease the activities of these muscles, neurotoxin is often required, and Letibotulinumtoxin A is the new addition to the pre-existing arsenal. It is derived from Clostridium botulinum strain CBFC26 and previously used for non-cosmetic purposes. However, it has recently been shown to have similar efficacy to ONA in treating glabellar lines and crow’s feet11.
In a prospective, randomised, parallel-group, double-blind, multicentre placebo-controlled phase III clinical trial, 355 participants with moderate to severe glabella frown lines received injections of 20 units (U) of either letibotulinumtoxin A or placebo. Interestingly, the response to treatment was defined as glabellar line severity (GLS) score of 0 or 1 (assessed by the subject and the investigator) and an improvement at week 4 of ≥2 points in GLS score relative to baseline (Figure 1). Remarkably, the results at 4 weeks showed a 78.6% response rate based on investigator’s assessment and 68.6% based on the subject’s assessment resulting in a composite responder rate of 64.7% for the active treatment group, whereas the placebo group had 0.00% response rate (p<0.001). Even more strikingly was the substantial improvement in GL severity which was reported as early as day 2, with the median time to onset of effect being 3 days and duration of treatment effect up to 127.26 days11. Surprisingly, no antibodies were detected during the study period even after repeated treatment and the safety profile of letibotulinumtoxin A even after repeated injection remained excellent11.
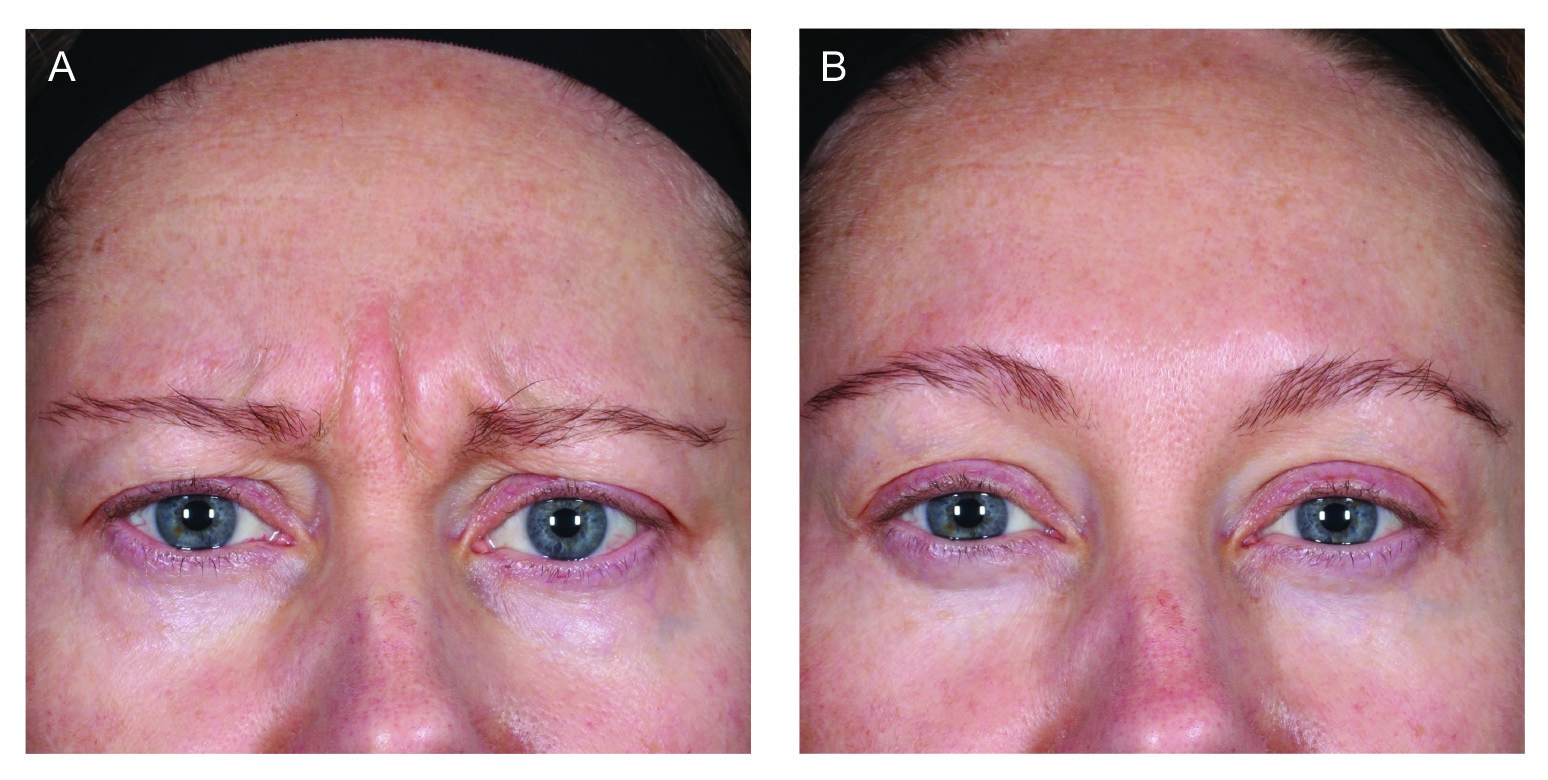
Figure 1. Before and after images of a female subjects at (A) baseline and (B) after 4 weeks11.
It is important to note that hyperfunctional glabellar frown lines can transmit facial miscues that adversely affect emotional communication, increasing perception of age and diminishes self-esteem. Therefore, Cox et al., (2024) evaluated the efficacy of letibotulinumtoxin A in mitigating the negative psychological impact associated with moderate to severe glabellar lines and assessed the subject satisfaction with treatment outcome in BLESS phase 3 clinical trials4. Conspicuously, among 1,272 participants, 85.5% had moderate to severe psychological impact at baseline and these were significantly improved by letibotulinumtoxin A. The study reported that attenuation of psychological burden was highly correlated with the improvement in glabellar line severity (p<0.0001). The conclusion reached here was that letibotulinumtoxin A significantly improved psychological burden associated with glabellar line across all trials and subjects experienced an improve quality of life (QoL), younger perceived age, and satisfaction with treatment outcome4.
Apart from psychological satisfaction, is Letibotulinumtoxin A effective in different age groups since a recent survey by the International Society of Plastic Aesthetic Surgeons (ISAPS) has suggested that most patients treated with BoNT-A are females aged between 35 to 501. A post hoc analysis of the phase 3 BLESS III study of Gold et al., (2024) evaluated the variation of treatment efficacy of letibotulinumtoxin A for treating vertical glabellar lines between in females aged 35 to 50 and those <30 or >50 years. The study included a total of 355 participants with moderate-to-severe glabella front lines, randomised to receive either 20 U of letibotulinumtoxin A or placebo1. The study found that composite responder rate for patients aged 35 to 50 receiving active treatment were significantly higher than the remaining female population receiving active treatment at weeks 1, 2, and 4. More notably was the higher rates of GLS improvement of ≥1 at weeks 1, 2, 4, 8, 12, 16, and 20 noted in females aged 35 to 50 on active treatment (Figure 2). The conclusion reached here was that the response rate for females aged 35 to 50 was higher at weeks 1, 2 and 4. Furthermore, the response rate remained higher up to week 16 with demonstrated treatment efficacy and safety in treating vertical glabellar lines in these female age groups1. In summary, letibotulinumtoxin A proves to be a new addition to the current cosmetic treatment for glabellar lines with remarkable efficacy and safety that is comparable to other formulation available in the market.
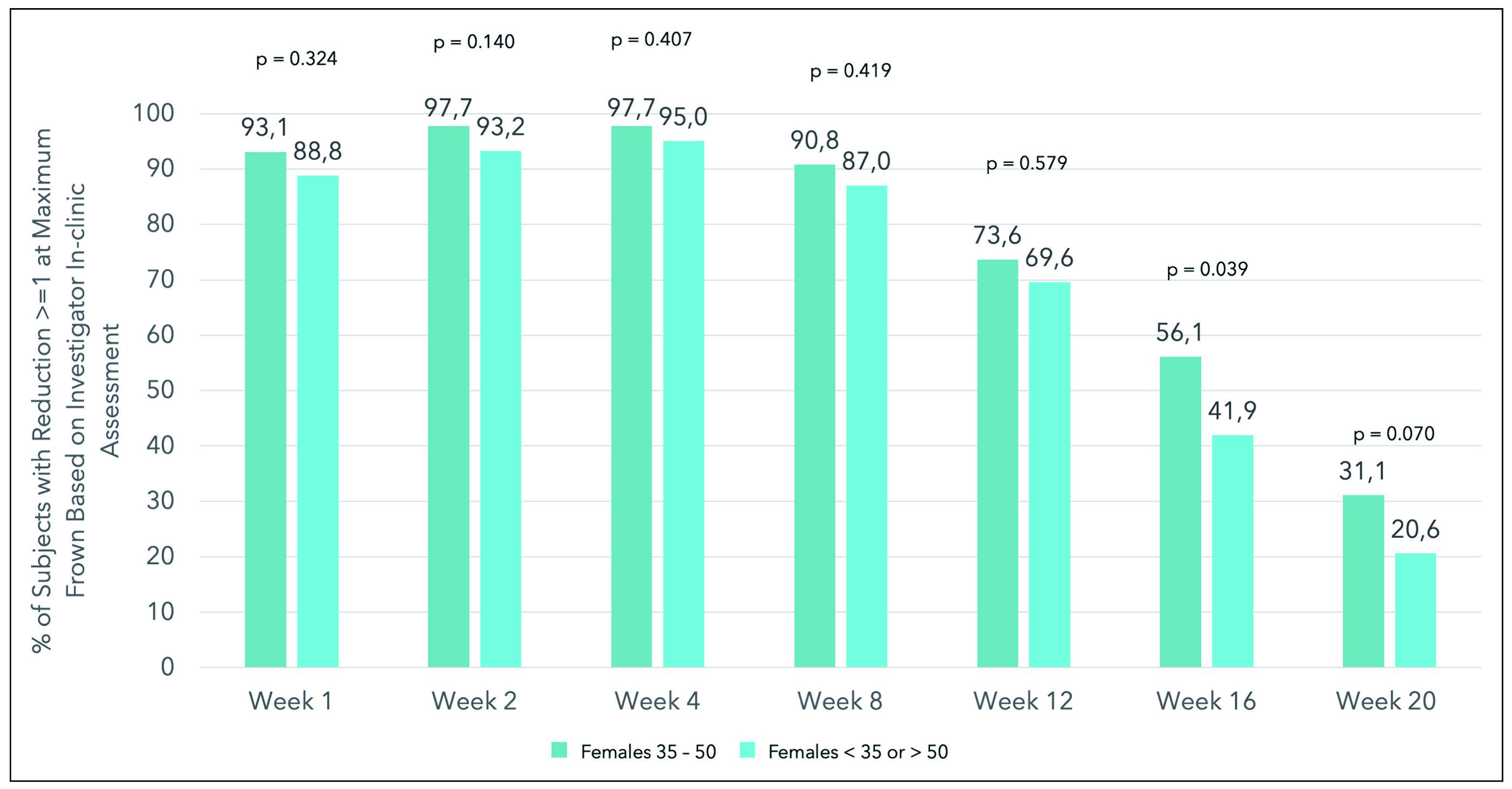
Figure 2. The percentage of patients with reduction ≥1 at maximum frown based on investigator in-clinic assessment for females aged 35 to 50 years and younger than 35 or older than 50 years1.
References
1. Gold M, et al. Aesthet Surg J Open Forum 2024; 6: ojae010. 2. Punga AR, et al. Clin Neurophysiol Pract 2023; 8: 169-73. 3. Ho WWS, et al. Plast Reconstr Surg Glob Open 2022; 10(6): e4407. 4. Cox SE, et al. Dermatologic Surgery 2024: 10.1097/DSS.0000000000004152. 5. Dover JS, et al. Dermatol Surg 2018; 44(2): 249-60. 6. Prager W, et al. Dermatol Surg 2017; 43(7): 959-66. 7. Ho WWS, et al. Toxins (Basel) 2023; 15(7). 8. Nassif AD, et al. Toxins (Basel) 2022; 14(2). 9. Obagi S, et al. J Drugs Dermatol 2017; 16(9): 929-30. 10. He X, et al. Molecules 2023; 28(14). 11. Mueller DS, et al. Aesthetic Surgery Journal 2022; 42(6): 677-88.





