
Associate Consultant,
Queen Mary Hospital
Honorary Clinical Assistant Professor,
The University of Hong Kong

Associate Consultant,
Prince of Wales Hospital
Honorary Clinical Assistant Professor,
The Chinese University of Hong Kong
Chronic kidney disease (CKD) is currently one of the most prominent causes of death and suffering, affecting >800 million individuals globally. Along with the rise in its risk factors, such as hypertension and diabetes mellitus (DM), the number of patients with CKD has been increasing in recent decades1. There is evidence to support the control of these risk factors to retard CKD progression2. In the symposium series titled “Optimising Medications for Hypertension and Diabetes to Maximise Kidney Care” co-organised by the Hong Kong Kidney Foundation, Hong Kong Society of Nephrology, and Hong Kong Association of Renal Nurses, Dr. Chan Chi Wang Gary and Dr. Fung Wing Shing Winston shared the essentials of pharmacological management to counter hypertension and DM, hence optimising renal protection.
While end-stage kidney disease (ESKD) substantially increases the risks of morbidity and mortality, Dr. Chan highlighted that less severe kidney dysfunction is also associated with suboptimal health outcomes. For instance, a community-based, longitudinal study involving 1,120,295 adults he United States (U.S.) by Go et al. (2004) indicated that decreasing levels of estimated glomerular filtration rate (eGFR) was associated with increasing risks of death, cardiovascular diseases (CVD), and hospitalisation (Figure 1A-C)3.
Figure 1. Age-standardised rates of A) death from any cause, B) CV events, and C) hospitalisation, according to the eGFR3
In estimating the risk of adverse outcomes, Dr. Fung emphasised that both eGFR and the severity of proteinuria have to be considered. "Relying only on the blood test showing a normal creatinine level without checking the proteinuria status would misrepresent the true risk and lead to missed opportunity in preventing kidney disease," he mentioned. An early appreciation of the patient’s kidney function facilitates timely treatment, whereas effective treatments for CKD are now available not only for delaying the progression of kidney disease but also for reducing the risks of related mortality and morbidity.
Given that hypertension and DM are independent risk factors of CKD, early detection and timely control of glycaemic levels and blood pressure can help prevent or delay CKD from progressing4. Clinically, there are 3 established pillars of therapy and a fourth emerging pillar in the management of diabetic kidney disease (DKD)5.
Based on the UKPDS 36 study (2000), each 10 mmHg decrease in mean systolic blood pressure was associated with reductions in the risk of any DM-related complication and DM-related deaths by 12% and 15%, respectively, in diabetic patients6. Accordingly, inhibitors of the renin-angiotensin system (RASi), including angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB), are widely employed to control the blood pressure of diabetic patients7.
The network meta-analysis by Palmer et al. (2015) suggested that ACEi and ARB were the most effective strategies against ESKD compared to other blood pressure-lowering agents (Figure 2)8. “RASi are superior to other antihypertensive agents in DKD for their capacity to reduce intraglomerular pressure by preferentially dilating the efferent arteriole7,” Dr. Chan explained. Nonetheless, due to the potential adverse effects of dual blockage with ACEi and ARB in DKD, he recommended that RAS inhibition is currently confined to a single agent.
Figure 2. Network meta-analysis of antihypertensive agents versus placebo for ESKD in adults with DKD8
As the second pillar of therapy, sodium-glucose cotransporter 2 inhibitors (SGLT2i) are able to reduce intraglomerular pressure and appear to attenuate the inflammatory and fibrotic responses of the human proximal tubular epithelial cells to high glucose. It is also able to enhance urinary glucose excretion to aid glycaemic control7.
Besides, cardiorenal protection by SGLT2i has been proven in various clinical trials. A meta-analysis involving data from 46,969 DM patients from 6 trials by McGuire et al. (2021) demonstrated that SGLT2is were associated with a reduced risk of major adverse CV events, hospitalisation for heart failure or CV death, and kidney outcomes, with no significant heterogeneity of associations with outcome9. The results reflected that SGLT2 inhibition can reduce CV morbidity and mortality as well as generate favourable kidney outcomes.
Over-activation of the mineralocorticoid receptor (MR) plays a vital role in the development of inflammation and fibrosis in the heart, kidneys, and vasculature, which in turn drives CKD and CVD progression. Hence, the blockade of MR offers a promising approach to control the progression of CKD and improve CV morbidity and mortality10. However, the application of steroidal mineralocorticoid receptor antagonists (MRA) was hindered by concerns about hyperkalaemia and worsening GFR. In this regard, Dr. Fung commented that non-steroidal MRA (ns-MRA) provide cardiac and anti-proteinuric benefits, with less profound effects on hyperkalaemia than steroidal MRAs.
Recent studies demonstrated that ns-MRA reduced the risk of clinically meaningful CV and kidney outcomes in DKD patients10. According to FIDELITY, which is the pooled analysis of 2 landmark trials evaluating the CV and kidney outcomes in ns-MRA-treated DKD patients, ns-MRA was associated with reduced risks of composite CV outcomes (hazard ratio [HR]: 0.86, p=0.0018) and kidney disease progression (HR: 0.77, p=0.0002, Figure 3), when compared to placebo among DKD patients across a range of GFR11.
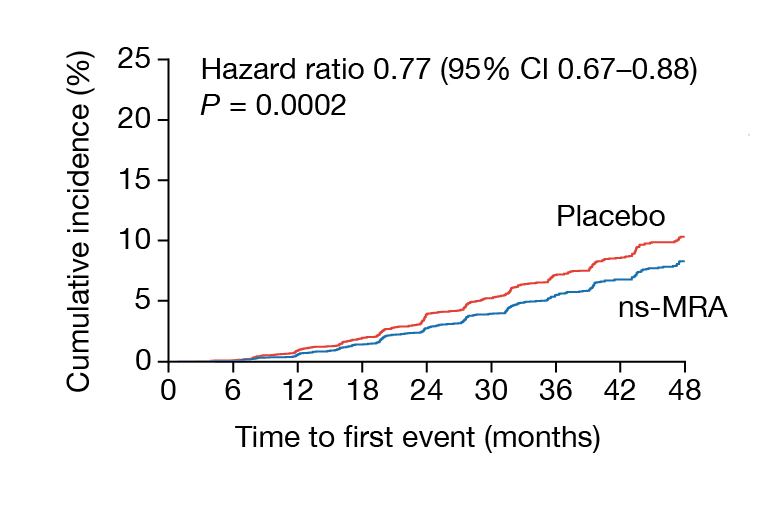
Figure 3. Composite kidney outcomes on patients treated with ns-MRA or placebo11
Dr. Fung added that ns-MRA showed modest effects on systolic blood pressure (SBP) and is generally well-tolerated by patients. Although hyperkalaemia increased, the related clinical impact was not significant (Figure 4)11. In view of the established efficacy, the KDIGO 2022 Guideline recommended ns-MRA for diabetic patients with eGFR ≥25 ml/min/1.73m2, normal serum potassium concentration, and albuminuria despite the maximum tolerated dose of RASi, whereas serum potassium should be monitored every 4 months if the concentration lies between 4.9 and 5.5 mmol/l12. Notably, Dr. Fung advised that suspending ns-MRA is needed if the serum potassium concentration rises over 5.5 mmol/l.
The glucagon-like receptor 1 receptor agonists (GLP-1RAs) were developed primarily for glycaemic control in diabetic patients. GLP-1RAs stimulate insulin release in response to glucose load through incretin release. By delaying gastric emptying and effects on the satiety centre in the brain, GLP-1RAs provoke weight loss5. Besides, GLP-1RAs have been shown to have CV benefits, such as reducing the risk of major adverse cardiac events. Essentially, secondary kidney outcomes in the CV outcome trials have suggested the kidney benefits of GLP-1RAs13.
A meta-analysis by Sattar et al. (2021) included 8 trials comprising 60,080 DM patients treated with GLP-1RAs, indicated that GLP-1RAs reduced all-cause mortality (HR: 0.88, p=0.0001), hospital admission for heart failure (HHF, HR: 0.89, p=0.013), and the composite kidney outcome (HR: 0.79, p<0.0001), with no increase in risk of severe hypoglycaemia, retinopathy, or pancreatic adverse effects14. Dr. Chan commented that the results of the FLOW study, which determines if GLP-1RAs demonstrate renal benefits for DKD patients15, are eagerly awaited.
Based on the existing clinical evidence, the KDIGO 2022 Guideline recommended a long-acting GLP-1RA for patients with type 2 DM and CKD who have not achieved individualised glycaemic targets despite the use of metformin and SGLT2i or who are unable to use those medications12.
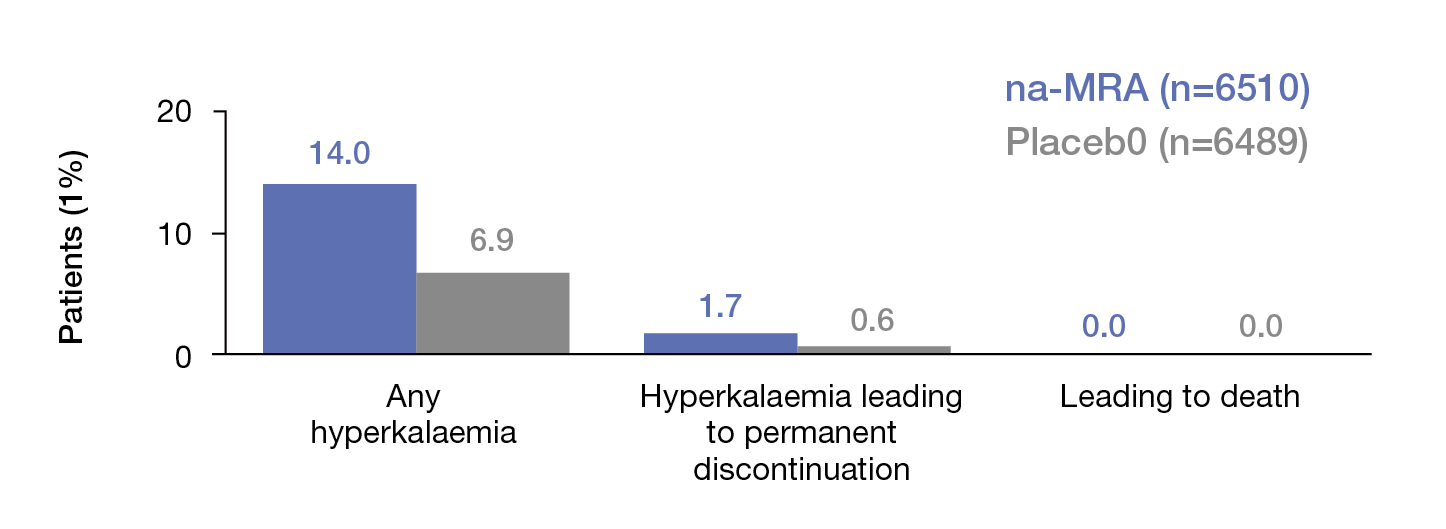
Figure 4. Clinical impact of hyperkalaemia due to ns-MRA and placebo11
Dr. Fung noted that RASi remains a cornerstone in managing CVD and CKD. Yet, the use of the medication may be limited by its potential to cause hyperkalaemia16. Nonetheless, the analysis of 205,108 patients with various comorbidities by Epstein et al. (2015) revealed that down-titration or discontinuation of RASi was associated with increased mortality across patient subgroups (Figure 5)16.
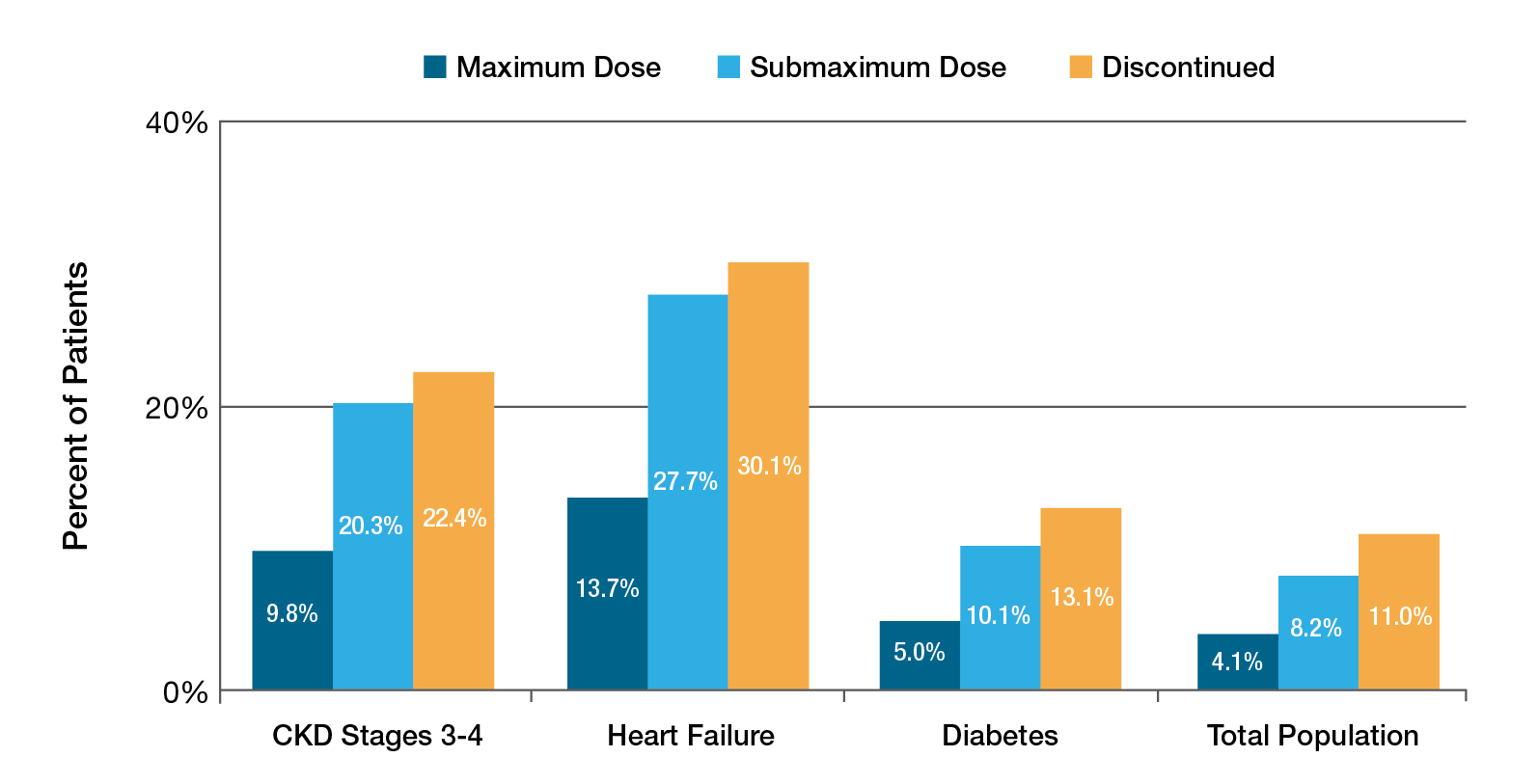
Figure 5. Mortality by prior RASi dose16
More recently, the STOP ACEi trial, which involved 411 patients with advanced and progressive CKD, by Bhandari et al. (2022) reported no significant difference in the decline in least-squares mean eGFR between discontinued or continued RASi therapy (Figure 6)17. Thus, Dr. Fung commented that RASi therapy in advanced CKD should be continued for their cardiorenal protective effects. The KDIGO 2024 Guideline advocated that hyperkalaemia associated with using RASi can often be managed by measures to reduce the serum potassium levels rather than decreasing the dose or stopping RASi18.
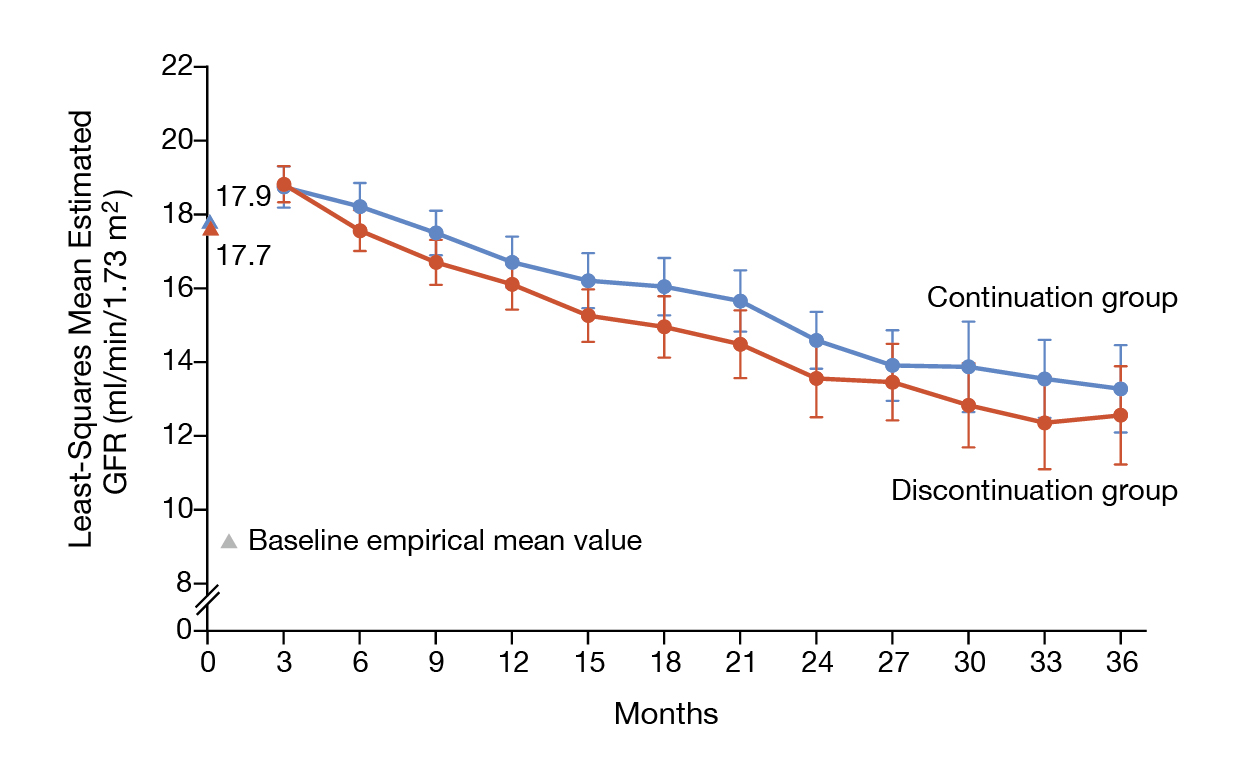
Figure 6. The eGFR over the 3-year follow-up in STOP ACEi trial17
The renal benefits of SGLT2is have been well-established in clinical trials. For instance, the EMPA-KIDNEY trial, which included 6,609 CKD patients, indicated that, after a median of 2 years of follow-up, SGLT2i treatment led to a lower risk of kidney disease progression or death from CV causes when compared to placebo19. Dr. Chan reminded us to be weary of the potential side effects of euglycaemic diabetic ketoacidosis and urinary tract infections associated with SGLT2i use.
Given the renal benefits of SGLT2i, Dr. Fung quoted that the KDIGO 2022 Guidelines advocated treating patients with DM, CKD, and an eGFR ≥20 ml/min/1.73m2 with an SGLT2i, particularly patients with heart failure or proteinuria12. For patients on SGLT2is, Dr. Fung addressed that patient education on early recognition and the implementation of the sick day protocol is essential to mitigate diabetic ketoacidosis, whereas self-examination of foots for wound by patients or caregivers is recommended.
Dr. Chan highlighted that patients with DKD should be treated with a comprehensive approach to improving both kidney and CV outcomes. This approach should include lifestyle modification and self-management. Notably, he added that lipid control is also important to abate excessive CV risk.
Dr. Fung summarised that early recognition of CKD is crucial. Screening for urinary protein and eGFR tests are recommended to identify patients who are at risk. For medication management, he suggested to consider starting with RASi and optimising the dosage to the maximum dose as tolerated. He emphasised that discontinuation is only a last resort. SGLT2i should be added, regardless of DM status. Moreover, prescribing ns-MRA and GLP-1RA for diabetic patients can be considered to delay kidney function decline. “With the information on optimising medication for kidney disease, we hope to treat the right patient at the right time with the right drugs to reach the right target,” Dr. Fung concluded.
References
1. Kovesdy. Kidney Int Suppl (2011) 2022; 12: 7–11. 2. Palmer et al. Lancet 2015; 385: 2047–56. 3. Go et al. New England Journal of Medicine 2004; 351: 1296–305. 4. ADA. Diabetes, High Blood Pressure, and Chronic Kidney Disease (CKD). 5. Agarwal and Fouque. Nephrol Dial Transplant 2023; 38: 253–7. 6. Adler et al. BMJ 2000; 321: 412–9. 7. Chan and Tang. Nephrol Dial Transplant 2016; 31: 359–68. 8. Palmer et al. Lancet 2015; 385: 2047–56. 9. McGuire et al. JAMA Cardiol 2021; 6: 148–58. 10. Georgianos and Agarwal. Kidney Int Rep 2021; 6: 2281. 11. Agarwal et al. Eur Heart J 2022; 43: 474–84. 12. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2022; 102: S1–127. 13. Shaman et al. Circulation 2022; 145: 575–85. 14. Sattar et al. Lancet Diabetes Endocrinol 2021; 9: 653–62. 15. Rossing et al. Nephrol Dial Transplant 2023; 38: 2041–51. 16. Epstein et al. American Journal of Managed Care 2015; 21: S212–20. 17. Bhandari et al. New England Journal of Medicine 2022; 387: 2021–32. 18. Stevens et al. Kidney Int 2024; 105: S117–314. 19. EMPA-KIDNEY Collaborative Group. New England Journal of Medicine 2023; 388: 117–27.





