Herpes zoster (HZ), also known as shingles, is caused by the reactivation of Varicella-zoster virus (VZV) that commonly leads to chickenpox. Normally, VZV remains dormant in the dorsal root ganglia or the sensory ganglia of the cranial nerve after a previous varicella infection. Varicella commonly affects children while herpes zoster occurs in adults or the elderly. It is believed that herpes zoster occurs due to the failure of the immune defence system to control the latent replication of the virus1. In immunocompromised individuals, the latent VZV can be reactivated, and cause local inflammation, and pain secondary to inflammation of the affected nerves with the virus. The incidence of VZV ranges from 1.2 to 3.4 per 1000 persons per year among younger healthy individuals, while incidence is 3.9 to 11.8 per 1000 persons per year among patients older than 65 years1. Locally, the prevalence of chickenpox is approximately 25.56 per 100,000 individuals, with only 1,987 cases reported officially2. Considering acquired VZV infection is common, the risk of developing HZ remains as studies have shown 1 in 3 individuals will develop HZ during their lifetime3. Even though most individuals suffering from HZ often have uneventful recovery, some may suffer from serious complications and sequela that may require hospitalisation4. Therefore, it is essential to understand the risk posed by HZ.
Immunodeficiency remains a substantial risk factor in the reactivation of VZV. Data from a meta-analysis by F. Marra in 2020 showed HIV/AIDS patients were 3 times more likely to develop HZ (relative risk [RR] = 3.22; 95% confidence interval [CI], 2.40–4.33), followed by patients with malignancies, such as lymphoma and leukaemia, (RR= 2.17; 95% CI, 1.86-2.53). Moreover, HZ was more prevalent in the older population (RR= 1,65, CI, 1.70 – 3.60) and those with a family history of HZ related infections (RR=2.48, 95% CI, 1.70 – 3.60).5
From 2020 to 2023, over 760 million individuals were infected by the coronavirus disease 2019 (Covid-19)6. Interestingly, as the number of Covid-19 cases rose, there was a concurrent rise in HZ cases. In fact, in Brazil, the incidence of HZ increased by 35.4% between March and August 2020 compared to the same period in 2017 and 2019. The trend was consistent in all age groups7. Similar findings were reported in a large retrospective cohort study conducted in the United States (US) between March 2020 and February 2021 in patients over the age of 50. The study included 394,677 Covid-19 positive patients (Covid-19 group) and 1,577,346 participants who were never diagnosed with covid-19 (non-Covid-19 group). Results from the study suggested that the Covid-19 group had a 15% higher risk of developing HZ compared to the non-Covid-19 group8. Among the Covid-19 group, those requiring hospitalisation for Covid-19 treatment were at greater risk of developing HZ (HR = 1.21, 95% CI 1.03-1.41)8. But what is the relationship between Covid-19 and HZ?
Well, experts have attempted to explain this phenomenon. The most common explanation derived was that Covid-19 may induce a significant reduction in T-cell, causing lymphopenia. Furthermore, the CD4+ T cell, CD8+ T cell, B cell and NK cell counts were much lower in Covid-19 patients compared to healthy individuals7. Why? The cell count change could be a result of the CD4+ helper and regulatory T-cell functional impairment, which may then lead to the hyperactivation, and subsequently the exhaustion of CD8+ T cells. Nevertheless, determining which Covid-19 variants and the degree of disease severity linked to shingles is often difficult since there are no studies available that evaluated the relationship between Covid-19 infection and shingles8.
It may be natural to ask if Covid-19 infections predispose individuals to develop HZ, what about the Covid-19 vaccinations? In fact, studies have shown the link between Covid-19 vaccination and HZ incidence. In a post-surveillance study conducted during the research on the BNT162b2 vaccine, a slight increase in HZ incidence was reported (RR 1.07; 95% CI 0.85–1.35)9. However, in a real-world study performed in Israel, the risk of VZV reactivation was shown to be 43% higher among mRNA vaccine recipients (RR = 1.43, 95% CI 1.20 – 1.73)10. An even greater risk was observed between Covid-19 vaccination and HZ during the analysis of the TriNetX database performed by M. Hertel and his team. Vaccinated and unvaccinated patients were divided into two cohorts, each with about 1 million patients. They found that the risk of HZ was 0.2% in the vaccinated group compared to 0.11% in the unvaccinated group (RR = 1.802, 95% CI 1.680 – 1.932, P<0.0001)11. Given that weakened immunity is a prime trigger for VZV reactivation and subsequent HZ infection, Psichogious proposed that the VZV-specific CD8+ T-cells may temporarily be incapacitated after covid vaccination, thereby increasing chances of VZV reactivation, and HZ. To summarise, the trigger of VZV reactivation after Covid-19 infection and Covid-19 vaccination stems from a similar impairment of immune cells, such as T-cells, as highlighted above.
Coronary artery disease (CAD) is also a risk factor for HZ. In a Taiwanese study, the risk of HZ was around 20% - 30% higher in CAD patients with type 2 diabetes mellitus (T2DM) compared with T2DM patients without CAD12. Therefore, CAD may contribute to an additional risk of HZ occurrence in adults with T2DM, but its role in non-diabetics remains elusive. However, in a separate meta-analysis, cardiovascular-related co-morbidities were shown to increase the risk of VZV reactivation (RR 1.34, 95% CI 1.17 – 1.54)5. It is believed that CAD was associated with a compromised adaptive immune response, affecting not only CD4+ T-cells, but also the CD8+ T-cells12.
Since T-cell immunity is the key to defending against VZV reactivation, a study was performed to assess the precise mechanisms related to this T-cell response in CAD population. In fact, in CAD population, monocytes differentiate into pro-inflammatory effector macrophages, which then cause a higher glucose uptake and increased mitochondrial activity, and glycolytic intermediates. Together, these reactions lead to enhanced transport of pyruvate (Figure 1). The resultant mitochondrial stress and generation of reactive oxidation species cause a sustained production of BMP4, eventually leading to an overexpression of immunoinhibitory ligands, programmed death ligand-1 (PD-L1) on the macrophage. Subsequently, the high density of PD-L1 on the surface of the macrophages sends negative signals to programmed death-1+ (PD-1+) T-cells, thereby weakening the T-cell activity. The proposed mechanism of impaired T-cell immunity may help explain the trigger of defective VZV-T-cell immunity, as well as VZV reactivation13.
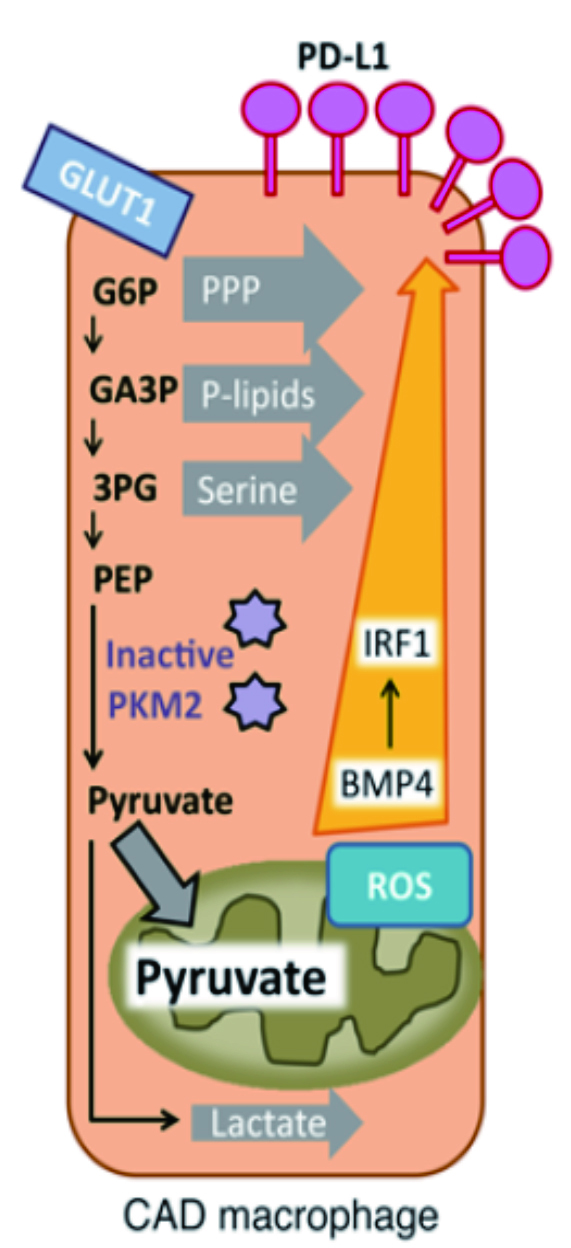
Figure 1. CAD macrophage. Excessive glycolytic intermediates generate higher concentration of reactive oxidation species. The resultant oxidative stress led to overexpression of PD-L1 on the surface of macrophages.
Despite the well-documented clinical correlation between CVD risk and HZ, their causal relationship may be mutual. Studies found that patients with HZ history have a higher chance of developing cardiovascular diseases. In a study by Curhan et al., involving 79,658 females in the NHS (2000-2016), 93,932 females in the NHS II (2001-2017), 31,440 males in HPFS (2004-2016) accounting for more than 2 million person-years on the longitudinal association of HZ and long-term risk of stroke or CVD, HZ was significantly and independently associated with higher long-term risk of CVD. Compared with individuals with no history of HZ, individuals with 5 to 8 years of history of HZ had 16% higher risk of developing coronary heart disease (CHD), 26% higher risk of CVD, and 38% risk of stroke14. Over the 13 years of study, while most of the cardiovascular events happened within 5-8 years since HZ, the risk may persist for 12 years or more since a heightened risk was found even after 13 years14. To explain these findings, authors attributed the elevated CVD risk to the vasculopathy triggered by VZV infection. In fact, VZV vasculopathy leads to alteration in the internal elastic lamina, intimal thickening, and a reduction in medial smooth muscle cells, thus lowering the arterial calibre and contractility (Figure 2). Moreover, HZ-related systemic inflammation, autoimmune reactions or haemodynamic perturbations may contribute to CVD events, in addition to disrupting the atheromatous plaques and coagulation cascade14.
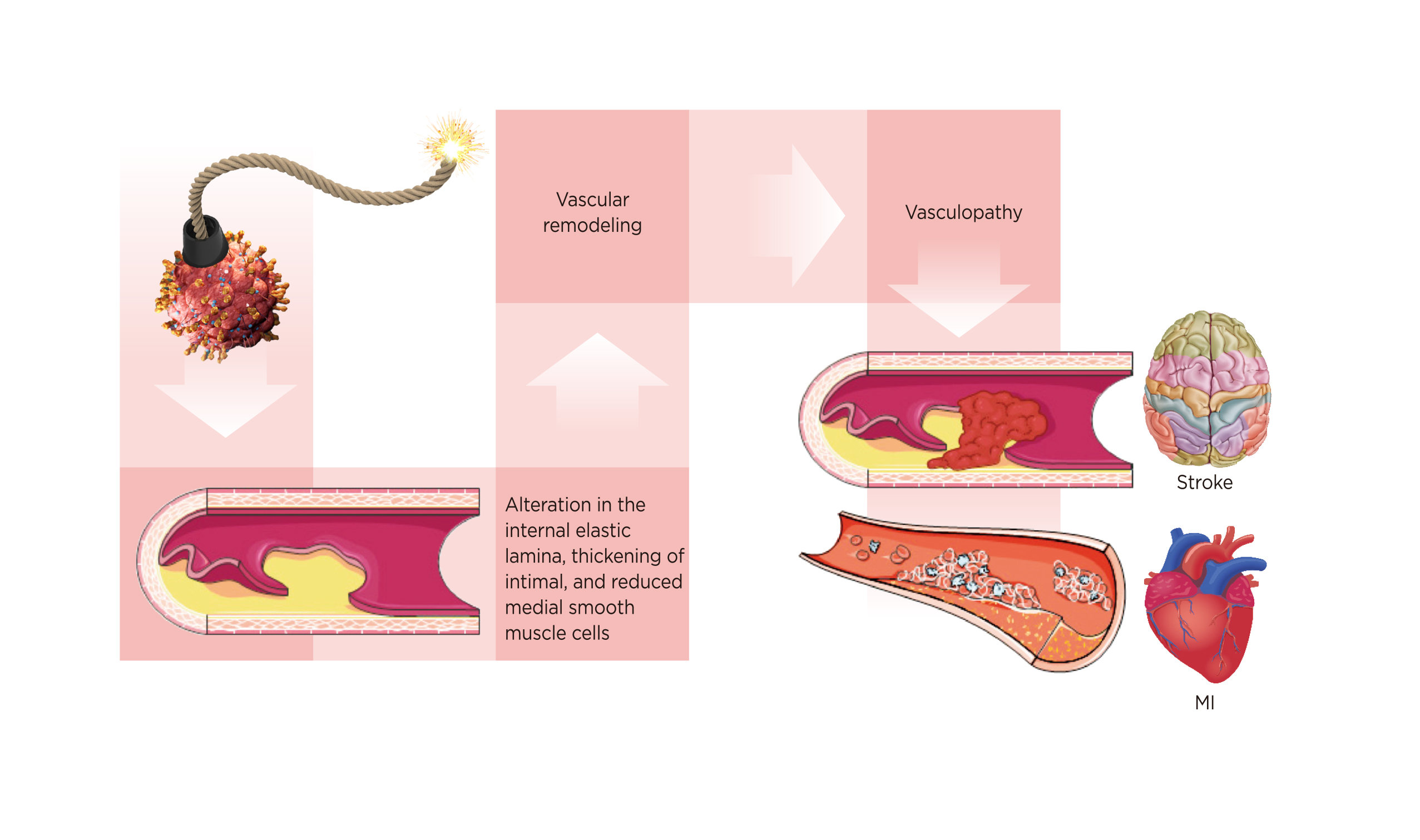
Figure 2. Development of vasculopathy after VZV reactivation14
Having considered the possible relationship between compromised immunity and HZ, the investigators performed an additional stratified analysis that compared the association of HZ and long-term risk of stroke and CHD among those with/ without immunocompromising condition. The analysis showed the risk of stroke is higher among immunocompromised females after 5 years14; however, the differences were statistically significant only in the NHS II cohorts but not in NHS cohorts (P interaction = 0.05). The results suggested that immunodeficiency is an additional risk factor for CVD in patients with HZ history.
Individuals who develop shingles should receive prompt treatment to alleviate symptoms and promote healing since the pain can often be debilitating15. Antiviral medications such as acyclovir or valacyclovir are commonly used to reduce the severity and duration of symptoms, in conjunction with the nonsteroidal anti-inflammatory drugs (NSAIDs) to reduce pain.
In conclusion, to mitigate the risk of developing shingles, we should consider a healthy lifestyle consisting of regular exercise, a balanced diet, and adequate sleep, all of which can help keep our immune system healthy. HZ vaccination may serve to keep VZV at bay and prevent VZV activation and HZ. The unmet needs of current treatment for HZ will be fulfilled in the near future through active research; nonetheless, prevention is often the best remedy for any surprise ambush by the VZV.
References
1. Nair PA, Patel BC. Herpes Zoster. StatPearls 2022; published online Sept 5. https://www.ncbi.nlm.nih.gov/books/NBK441824/ (accessed May 15, 2023). 2. Hong Kong Department of Health. Health Facts of Hong Kong Population and Vital Statistics for 2020. 2021 Edition. 3. Clinical Overview of Herpes Zoster (Shingles) | CDC. https://www.cdc.gov/shingles/hcp/clinical-overview.html#:~:text=Approximately%201%20out%20of%203,however%2C%20multiple%20episodes%20are%20possible (accessed May 15, 2023). 4. Patil A, Goldust M, Wollina U. Viruses 2022; 14. DOI:10.3390/V14020192. 5. Marra F, Parhar K, Huang B, Vadlamudi N. Open Forum Infect Dis. 2020; 7. DOI:10.1093/ofid/ofaa005. 6. WHO. WHO COVID-19 Explorer. 2023. https://covid19.who.int/ (accessed March 30, 2023). 7. Algaadi SA. Infection. 2022; 50: 289–93 8. Bhavsar A, Lonnet G, Wang C, et al. Open Forum Infect Dis 2022; 9. DOI:10.1093/ofid/ofac118. 9. Shasha D, Bareket R, Sikron FH, et al. Clinical Microbiology and Infection 2022; 28: 130. 10. Barda N, Dagan N, Ben-Shlomo Y, et al. New England Journal of Medicine 2021; 385: 1078–90. 11. Hertel M, Heiland M, Nahles S, et al. Journal of the European Academy of Dermatology and Venereology 2022; 36: 1342–8. 12. Ke CC, Lai HC, Lin CH, et al. PLoS One 2016; 11. DOI:10.1371/journal.pone.0146750. 13. Watanabe R, Shirai T, Namkoong H, et al. Journal of Clinical Investigation 2017; 127: 2725 -38. 14. Curhan SG, Kawai K, Yawn B, Rexrode KM, Rimm EB, Curhan GC. J Am Heart Assoc 2022; 11. DOI:10.1161/JAHA.122.027451. 15. Johnson R, McElhaney J, Pedalino B, Levin M. Int J Infect Dis 2007; 11 Suppl 2. DOI:10.1016/S1201-9712(07)60021-6.





